Please note that this material is copyright to the publishers of the Journal of Interdisciplinary Cycle Research and to the authors
Not to be reproduced in any way without permission except for personal study

Journal of Interdisciplinary Cycle Research, 1992, Vol. 23, No. 2, pp. 128-135
Studies on Halimeda IV.
An Endogenous Rhythm of Chloroplast Migration In the Siphonous Green Alga,
H. distorta*
by
Edward A. Drew and Kay M. Abel
Australian Institute of Marine Science, PMB No. 3, M.S.O., Townsville, Queensland 4810, Australia*Contribution No. 737 from the Australian Institute of Marine Science
Abstracting keywords: Halimeda, coenocytic green alga, chloroplast movement, circadian rhythm, continuous light, continuous dark.
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
ABSTRACT
Halimeda has been found particularly suitable for studies of long-distance chloroplast migration by virtue of its coenocytic structure and calcium carbonate skeleton. A circadian rhythm of chloroplast migration in Halimeda distorta was monitored by videography of segment surface pigmentation. In normal 12 h light/12 h dark treatments synchronised with dawn and dusk, the segments were green all day, began to become pale immediately the light was turned off, and then remained almost white for most of the night until beginning to re-green a few hours before dawn. As a result of that, they were already quite green by the time the light was turned on. In continuous darkness a similar cycle, albeit with reducing amplitude and a period of about 23 hours, was maintained for at least 7 days. However, this cycle differed significantly from the normal one in that the segments did not remain green after the light was not switched on at dawn, but rather began to pale immediately thereafter. Conversely, in continuous light the segments did not become pale at any time. Thus, the rhythmical re-emergence of the chloroplasts before dawn and their subsequent withdrawal appears to be controlled by an endogenous rhythm which is independent of light. However, light does completely, but reversibly, inhibit the chloroplast withdrawal component of the cycle. This behaviour of the chloroplasts in Halimeda is very similar to that in the related alga, Caulerpa, but it is quite different from that in another extensively studied but unrelated siphonous green alga, Acetabularia, in which the circadian rhythm of chloroplast migration is maintained in continuous light.
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
INTRODUCTION
Rhythmic changes in chloroplast distribution within macroscopic, siphonous (coenocytic) green algae have been reported by Dawes & Barilotti (1969) in Caulerpa, Koop et al (1978) in Acetabularia and Drew & Abel (1990) in Halimeda. All three algae exhibited basipetal migration associated with darkness during 12h light/1 2h dark cycles. The endogenous nature of these rhythmical chloroplast migrations was clearly demonstrated by their persistence for at least 7 days in Acetabularia maintained in continuous light, and for at least three day in Caulerpa in continuous darkness. The observation that acropetal migration of chloroplasts began several hours before dawn in Halimeda also suggests control by an en�dogenous rhythm not directly related to the alternation of light and dark periods.
Halimeda is particularly suitable for in vivo studies of chloroplast movement because the segments demonstrate dramatic colour changes from green to white which Drew & Abel (1990) showed to be due large scale redistribution of the chloroplasts from the primary utricles at the surface of the segments into the medullary filaments beneath the calcium carbonate skeleton (Figure 1 B). This is only possible because the plant is coenocytic, lacking any crosswalls within its branching filamentous structure. The chloroplasts probably move along networks of microtubule/actin filaments similar to those described by Menzel & Schliwa (1986 a,b) in the related alga, Bryopsis. In this paper we describe experiments using a video camera and computer-assisted data analysis to record changes in surface pimentation during 7-day treatments in continous darkness, continuous light and a 12 h light/l2 h dark regime.
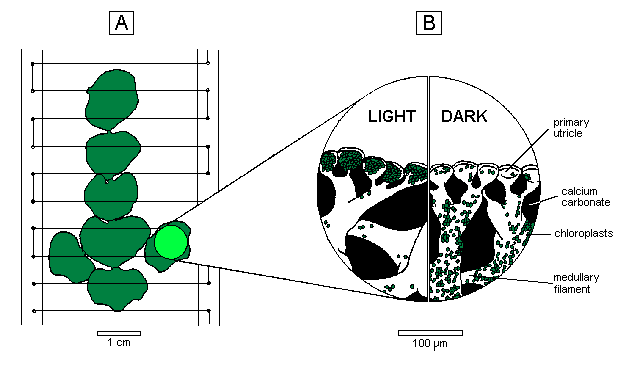 |
|
A: Excised branch tip supported by nylon filaments stretched across a perspex frame. The circle on one segment represents the 8 mm diameter area analysed for surface pigmentation; plant tip oriented downwards to mimic the pendulous habit of Halimeda. B: Distribution of chloroplasts in the light and dark within the surface utricles and medullary filaments. |
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
MATERIALS AND METHODS
Halimeda distorta (Yamada) Colinvaux, collected from the northern Great Barrier Reef, was cultivated in the open-circuit seawater cascade described by Drew & Abel (1988). In each experiment four terminal branches, consisting of five or six healthy segments, were held in place on a vertical frame by a series of nylon threads (Figure 1 A) and incubated in filtered seawater as described by Drew & Abel (1990).
The experiments were carried out in a darkroom with illumination provided by a Hanimex 2555 slide projector. This was kept on continuously, and an opaque slide was inserted by the automatic slide changer to prevent illumination of the plants during dark periods. This procedure was necessary to allow video recording during dark as well as light periods. The pause function of a JVC GX-NE7 video camera linked to a JVC HR-S10A video cassette recorder (VCR) was controlled by an HX20 microcomputer so that 5-second sequences were recorded every 5 minutes. Although data was required only once every 30 minutes, this recording frequency was used because the VCR switched itself off if left on pause any longer. During the dark periods the HX20 also caused the opaque slide to be removed from the light path, and then immediately replaced, once every 30 minutes to produce a 300 millisecond light pulse synchronised with one of the recording pulses. In this way a series of 8 equally exposed video frames, together with about 30 seconds of darkness, were recorded every 30 minutes during the dark periods, and a series of 5-second sequences were recorded at 5 minute intervals throughout each light period.
The video tapes were analysed using a JVC HR-D725EA VCR, capable of displaying noise-free images on pause, and an Amiga 1000 computer equipped with a genlock device which permitted the video image and the computer output to be displayed simultaneously on the same monitor screen. To measure surface pigmentation of a segment, the appropriate video image was located, the VCR paused and then the part of the image to be measured was selected with the computer's mouse cursor and a circular black mask generated to obscur nearby bright parts of the image whilst leaving an 8 mm diameter circular area of segment visible at its centre. This represented 50% or more of the image of a single segment. A Licor quantum sensor was held against this circular area and the light intensity recorded at the screen surface was digitised, using a Westlog datalogger in A-D mode, and displayed directly on screen. Once this value had stabilised, a single key stroke caused the value and time-course identification data to be stored in a disk file. Since successive video images were always positioned in exactly the same place on the monitor screen, it was only necessary to advance the tape to the next time period, pause the VCR and repeat the measurement process.
Black and white standard panels were also included in each video frame but as these gave very consistent values for light intensity, it was necessary to measure them only at the beginning and end of analysis sequences. They were used mainly to standardise monitor settings between sessions. Values for the brightness of the segment images were expressed as percentages of the difference between the black and white values. This measure of surface pigmentation has been plotted so that the green, darker values showed as peaks and the paler segments as troughs.
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
RESULTS Normal response- Continuous darkness- Continuous light- Pre-dawn reversal
RESULTS AND DISCUSSION
The normal response
All experiments started and ended with one day under standard conditions of 12h light/12h dark periods in order to establish, initially, that the particular plants being used responsed as expected and, subsequently, to determine if the intervening treatments had any adverse effect which changed that response. The normal response described by Drew & Abel (1990) was that all the segments changed rapidly from green to nearly white within 2 hrs of the start of darkness, and then remained very pale for 6 or 7 hrs before starting to become green again 3 to 4 hrs before the light was due to be switched on at dawn. Pre-dawn re-greening progressed more slowly than the previous paling, and was usually not completed until an hour or more after the light period started. In these experiments the start of the dark and light periods coincided approximately with local dusk and dawn.
RESULTS Normal response- Continuous darkness- Continuous light- Pre-dawn reversal
Continuous darkness
This treatment was initiated at 0600 h on day 3 of the experiment by not switching the light on after a normal night. The expected dark pattern was duly completed with the segments quite green by the time the light period should have started. However, at the time when the light should next have been switched on but was not, the segments immediately began to go pale again, although at an even slower rate than they had re-greened. As a result, they were already quite pale by 1800 h when the next dark period would normally have started.
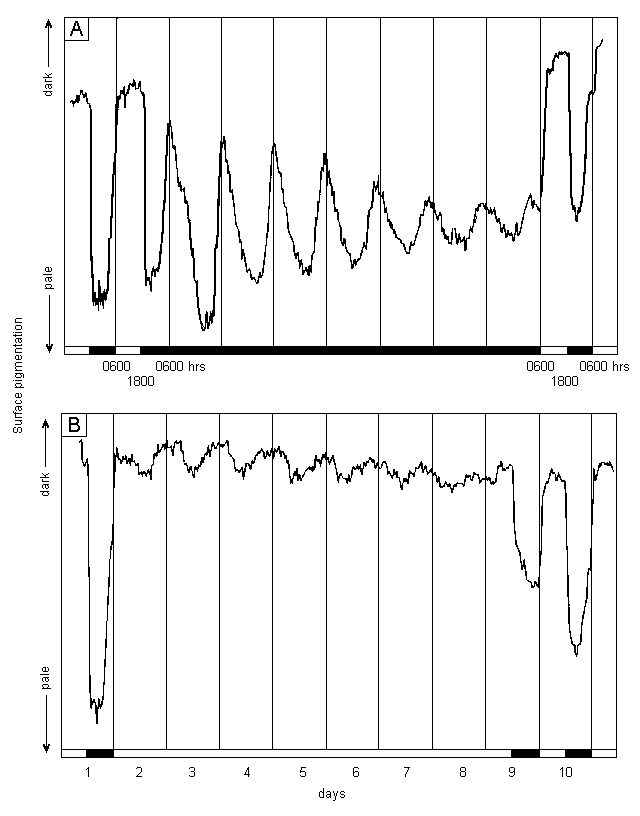
A very similar pattern was maintained for the next 6 days (Figure 2 A), although the amplitude of the colour changes became progressively reduced. In addition, the times of maximum and minimum surface pigmentation occurred progressively earlier, indicating a cycle of about 23 hours duration.
The normal light/dark cycle was then restarted at 0600 h on day 10 by switching the light on. Although the segments were at that time in the process of becoming pale, because of the 23 h cycle now in place, rapid greening began immediately and progressed to a level even greater than that observed prior to the extended dark period. The pattern the following night was indistinguishable from the normal response recorded at the start of the experiment.
This experiment indicates that the cyclic colour change is controlled by an endogenous rhythm with a period close to, but not identical with, the 24 h day. However, the other requirements for definition of a circadian rhythm, such as temperature compensation (Schweiger & Schweiger, 1977), still require to be tested. Therefore, this rhythm currently has the same status as that described by Koop et al (1978) in Acetabularia.
 |
|
A: In continuous darkness. B: In continuous light. Plants subjected to 1 day of 12h light/12h dark at the start and one such day at the end of the experiment; white and black bars indicate illuminated or dark conditions; when appropriate, daily sectors started at 0600 hrs (light on) and ended at 1800 hrs (light off); water temperature 25oC throughout, irradiance 100 mol photons m-2 s-1 PAR (photosynthetically active radiation - 400 to 700 nm) from tungsten halide projector lamp; incubation tank flushed with new filtered seawater only on days 1 and 11 to avoid possible entraining effects of regular flushing. |
RESULTS Normal response- Continuous darkness- Continuous light- Pre-dawn reversal
Continuous light
The endogenous rhythm in Acetabularia was detected in continuous light, not continuous darkness as described above for Halimeda. However, when Halimeda was exposed to continuous light, the rhythmical paling of segments was not detected (Figure 2 B). Rather than paling during the first omitted dark period, the segments actually became slightly greener, remained that colour for more than 12 hours, and then paled slightly. This sequence was repeated, with decreasing amplitude, throughout the 7 day period of continuous light, producing a series of small oscillations superimposed on the normal level of daytime pigmentation. These oscillations also had a period of about 23 hours.
As soon as dark periods were reinstated at 1800h on day 9, rapid paling commenced, although this proceeded only about half as far as that achieved on day 1. In addition, pre-dawn re-greening did not occur, indicating that 7 days continuous light had interferred with that part of the endogenous rhythm. Rapid re-greening to the expected daytime level commenced as soon as the light was switched on again and the pattern the following day was indistinguishable from the normal response recorded at the start of the experiment, including pre-dawn re-greening.
RESULTS Normal response- Continuous darkness- Continuous light- Pre-dawn reversal
Pre-dawn reversal of greening
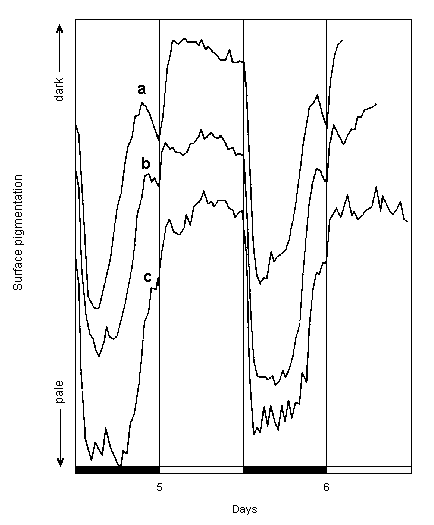
The tendency for chloroplast withdrawal to commence soon after pre-dawn emergence if the light did not come on, as observed in the continuous darkness experiment, was also observed in other experiments in which H. distorta was exposed to 12h light/12h dark cycles, particularly those involving pretreatment at 15 and 20oC. As shown in Figure 3, re-greening started many hours before the next light period in these experiments and the segments became quite green, and then starting to become paler again, well before the light was switched on at dawn. However, immediately the light was switched on, this pre-dawn paling was reversed and the segments became even greener than they had been at the pre-dawn maximum. Microscopical observations on other Halimeda species have shown that few of the re-emerging chloroplasts actually enter the primary utricles until after dawn (Drew, unpublished). This may be the furthest point the chloroplasts can reach in the dark, with the extra greening induced by light resulting from the chloroplasts actually passing through the narrow constriction at the base of each primary utricle and becoming even more visible as they congregate above the outermost layer of calcium carbonate.
 |
|
Graphs a - c show changes in surface pigmentation at 25oC on days 5 and 6 in experiments where plants had been subjected to 15, 20 and 25oC respectively during days 2 to 4; graphs are vertically offset for clarity; other details as for Fig. 2. |
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
CONCLUSIONS
Halimeda chloroplasts move between the primary utricles at the segment surface and the medullary filaments within the calcium carbonate skeleton according to an endogenous rhythm which is independent of light and has a 23 hour period. However, since the chloroplasts only remain at the segment surface for any length of time during periods of illumination, light must exert an strong inhibitory effect on either the process of chloroplast movement or the endogenous rhythm controlling that movement.
GO TO Title- Abstract- Introduction- Materials and Methods- Results and Discussion- Conclusions- References
REFERENCES
- DAWES, C.J. and BARILOTTI, D.C. (1969):
- Cytoplasmic organisation and rhythmic streaming in growing blades of Caulerpa prolifera.
Am. J. Bot. 56, 8-15.
- DREW, E.A. and ABEL, K.M. (1988):
- Studies on Halimeda. II. Reproduction, particularly the seasonality of gametangia production, in a number of species from the Great Barrier Reef Province.
Coral Reefs 6, 207-218.
- DREW, E.A. and ABEL, K.M. (1990):
- Studies on Halimeda. III. A daily cycle of chloroplast migration within segments.
Bot. Mar. 33, 31-45.
- KOOP, H.-U, SCHMID, R. H.-H. HEUNERT and MITLTHALER, B. (1978):
- Chloroplast migration; a new circadian rhythm in Acetabularia.
Protoplasma 97, 301-310.
- MENZEL, D. and SCHLIWA, M. (1986a):
- Motility in the siphonous green alga Bryopsis. I. Spatial organisation of the cytoskeleton and organelle movements.
Eur. J. Cell. Biol. 40, 275-285.
- MENZEL, D. and SCHLIWA, M. (1986b):
- Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organised arrays of both microtubules and actin filaments.
Eur. J. Cell. Biol. 40, 286-295.
- SHIMMEN, T. and YANO, M. (1985):
- Ca2+ regulation of myosin sliding along Chara actin bundles mediated by native tropomyosin.
Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 61, 86-89.
- SCHWEIGER, H.-G. and SCHWEIGER, M. (1977):
- Circadian rhythms in unicellular organisms: An endeavour to explain the molecular mechanism.
Int. Rev. Cytol. 51, 315-342.