An understanding of how lubricating systems work is crucial to the selection of a
lubricant for a particular application. This essay could be summarized in one sentence:
lubricants provide a protective film that separates the two rubbing surfaces and reduces
the level of friction in the two rubbing surfaces.
Any surface contains irregularities, even when polished to a mirror finish. These
irregularities may not be visible, except under a microscope. When two highly-polished
surfaces are brought gently together, only some points on the surfaces will make contact.
These contacts will be brought closer together when a force is applied at right angles to
the surfaces (this force is referred to as a �normal load�), and the number of contact
points will increase:
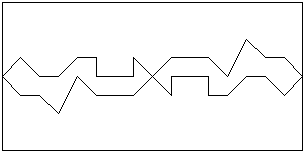 |
If a protective film were present on each of the surfaces, the surfaces could be separated:
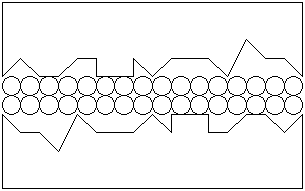 |
The protective film must adhere to each surface in order not to be sheared off or pushed
aside by the movement of the surfaces, particularly under a load.
The most commonly available lubricants today are manufactured from petroleum. A
typical lubricant molecule consists of a long chain of carbon and hydrogen atoms, called
an alkane:
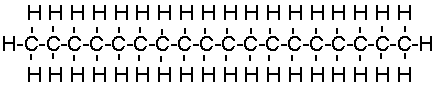 |
An oil usually has between 15 and 20 carbon atoms. A grease has between 20 and 25
carbon atoms. The hydrogen atoms form very weak bonds with the surfaces. In the cases
of brass and steel, metals consist of atomic nuclei surrounded by a sea of electrons. The
hydrogen atoms are bonded to the carbon atoms by a pair of shared electrons, which face
the carbon atoms: since there are no electrons on the other side of each hydrogen atom,
the other side has a slightly positive charge because of the hydrogen nucleus. This
positive charge attracts the hydrogen atoms to the sea of electrons of the metals. While
the bonding is very weak, the total bonding caused by a large number of hydrogen atoms
in a long carbon chain is considerably greater than in a short carbon chain. A shorter
carbon chain could therefore be removed more easily by the force acting upon the
surfaces. The forces that attract the molecule to the surface must be greater than the
forces that attract the molecule to other molecules: this makes it possible to have the
lubricant adhere to the surface yet easily slip over other lubricant molecules, and the
level of friction is reduced by having lubricant molecules rubbing against one another
rather than brass and steel surfaces rubbing together. This is as simple a description of
lubrication theory as you would find anywhere!
Longer carbon chains get tangled together like spaghetti, thereby making the ability of
the lubricant to flow more difficult. This is why lubricants with longer carbon chains are
thicker than lubricants with shorter carbon chains.
A lubricant with a longer carbon chain is less volatile. As the temperature increases, the
molecules vibrate more vigorously until the vibration causes the weak intermolecular
bonds to break. As they break, molecules become free to float away or evaporate.
Molecules with longer carbon chains vibrate less at a given temperature because they
have more mass, so they are less likely to evaporate. Situations in which a lot of heat is
generated, such as a car engine, require oils that will not evaporate easily. Bearings with
slow-moving parts do not experience such levels of heat, so a different lubricant is
required. A lubricant with larger molecules should be used to minimize evaporation,
particularly on bearing surfaces that are subjected to high torque and low speeds
(revolutions per minute). However, thicker lubricants can interfere with the action of the
mechanism when applied to bearing surfaces that are subjected to very low torque and
high speeds, so a thinner lubricant must be used, even though thinner lubricants are more
likely to evaporate! The purpose of application is particularly important in selecting a
lubricant because of the need to consider heat, torque and speed. If a shaft rotates very
quickly in a bearing, the fast motion has the effect of bringing more lubricant into the
bearing, called a hydrodynamic effect, which increases the separation of the rubbing
surfaces. Slower-moving applications do not benefit from the hydrodynamic effect.
You will have noticed that water boils at 100� Centigrade: at 99�, it is a
liquid; at 101�, it is a gas. Water freezes at 0�, again within a similarly
narrow range of temperatures. Oils, however, solidify gradually as the temperature
becomes colder, slowly becoming thicker and thicker until the oil no longer flows: oils
solidify over much wider temperature ranges because oils are manufactured as blends of
many different oil molecules mixed together to modify the lubricating and thickness
properties of the combined mixture. There are many different compounds with the same
number of carbon and hydrogen molecules, so while they have the same empirical
formula, such as C18H38 .
Their structures are all different, as are their lubricating and
thickness properties. Different compounds with the same empirical formula are called
isomers. Lubricants can be prepared with blends of isomers as well as with blends of
molecules of different lengths of carbon chain to further modify the properties of the
combined mixture, which solidifies over a wider range of temperatures and becomes a
solid at a lower temperature than an equivalent oil with just one type of molecule (one
isomer). Chemical engineers can therefore prepare a lubricant suited for each application,
or a lubricant suited for a wide variety of applications. This is called the Base Oil.
The major mineral oil components are paraffinic, naphthenic, and a smaller amount of
aromatic compounds. Paraffinic oils are straight chain (as in the example above) or
branched aliphatic hydrocarbons (in other words: alkanes and their isomers). Naphthenic
oils have saturated hydrocarbons (alkanes) that have at least one closed ring of carbon
atoms. Aromatic compounds have one or more benzene rings in each molecule. The
variations in composition directly affect the lubricating properties of the blended
mixture. Other chemicals are added to the base oil to improve the properties of the oil.
Mineral oils react with oxygen at hot temperatures to form hyperoxides, followed by
organic acids, which cause the formation of sludge and varnish and the corrosion of
metal parts. Chemicals called oxidation inhibitors are added to the base oils to prevent
the deterioration of the oil and the corrosion of metal parts. Similar chemicals called rust
inhibitors are added to protect steel parts. Dispersants and detergents are added to help
dissolve contaminants and sludge so that deposits are not formed. Other chemicals, such
as anti-wear and anti-scuff additives, that are sometimes added to base oils, react with the
metal atoms on the surfaces to form a protective layer that has a lower friction than that
of the metals rubbing against each other.
Applications that involve higher levels of torque and slower speeds (obliviating the hydrodynamic effect), require lubricants that can adhere more strongly to the metal surfaces. With weaker bonding, the lubricant molecules can be sheared off by the action of the surfaces, and these molecules must be replaced immediately by other molecules. Stronger bonding can be achieved on metal surfaces by using a lubricant that has polarized molecules (at one end), such as a metallic soap like lithium stearate. The polarized end of the molecule is attracted to the metal surfaces while the long alkyl group forms part of the protective blanket that covers the surface. The metallic soap will have a lower melting point than a comparable but nonpolarized alkane because of much stronger hydrogen bonding between the polarized molecules, compared to the much weaker (van der Waals) intermolecular forces. The hydrogen bonding between the molecules also strengthens the protective blanket that covers the surfaces. If you buy an automotive wheel-bearing grease that says 'lithium base' on the container, it would probably contain lithium stearate or a similar lithium soap.
Graphite is an excellent additive because its layers of atoms slide easily over one another.
Since graphite does not form bonds with the metal surfaces, it is easily lost: mixing the
graphite in base oils makes it possible to lower the friction level beyond the level
achievable by the oils alone, and the oils act as carriers, or as a medium to contain the
graphite.
Other lubricating techniques are used to separate rubbing surfaces, such as teflon
coatings, which act as dry lubricants to minimize friction and the drag caused by liquid
lubricants. The techniques are different, but the goals are the same: to separate rubbing
surfaces and to minimize friction.
Synthetic lubricants are manufactured for specific purposes. These lubricants are not
blended from natural oils, but rather produced artificially under controlled conditions to
minimize levels of contaminants. By having more control over what goes into a lubricant
product, chemical engineers hope to produce a superior lubricant: ideally, they want a
thinner lubricant with a higher boiling point (and therefore low evaporation rate), no
thermal breakdown of the oil molecules, and with higher lubricity (a measure of the
extent to which friction is reduced).
Before the advent of the automobile, petroleum was used mainly for manufacturing
kerosene for lamps. Most oil products were obtained from animal, plant and fish oils and
fats. These natural oils tend to contain mainly alkenes (unsaturated hydrocarbons), so
they differ with their alkane counterparts in that they have lower melting points: the same
lubricity could be achieved with a thinner oil, assuming both oils being compared had the
same number of carbon atoms. However, these oils tend to have fatty acids that must be
neutralized. Alkenes are not as stable as alkanes, so they are more easily oxidized into
fatty acids and they become more unstable when subjected to heat. These oils are not
hostile to bacteria, which accelerate the deterioration of the oils, whereas mineral and
synthetic oils have longer life expectancies. Fatty acids tend to corrode metal parts and
also to result in the formation of sludge. Oils and greases for clocks and watches were
(and many still are) made from fish oils, whale fat and porpoise oils being among the
favourites. The new oils have additives that protect the oils from bacteria and oxidation,
thereby extending their life expectancies considerably. The principles by which
lubrication takes place are the same, as outlined above. Note, however, that even the new
natural oils have essentially no tolerance for heat and must therefore not be used where
heat is generated (such as electric clock motors). You must use a mineral oil for electric
clock motors, such as a single-weight, non-detergent oil.
I hope this essay has made you a better-educated consumer of oils and greases for clocks,
electric motors, cars, or anything else. It is only a brief overview of a few aspects of tribology that might be of interest to horologists. Tribology, or the study of lubrication, is a very wide field: the more you know, the more you know you do not know! I prefer petroleum-based mineral oils over any
other, especially since the quality of mineral oils has improved to such an extraordinary
extent in just the last fifteen years, thanks to the hard work of many chemical engineers!
Most mineral oils manufactured and marketed in the United States by well known
manufacturers are of very high quality indeed, and a statement that the oil meets
government and manufacturer specifications is more reassuring still. However, you still
must be cautious when selecting lubricants since some lubricants are poorly engineered
and since many will not suit the particular application you wish to apply it to (the same is
true of clock oils from your clock suppliers: the fact that it says �clock oil� on the bottle
does not mean that the clock oil you bought is of high quality or that it will provide
adequate protection of the second wheel pivots, as it might for the escape wheel pivots).
Consider these examples. There is a very expensive clock oil that I have had many
problems with because it dries after about a year and a half. It is also very thin: it tends to
run too easily when applied to bushings. There is another clock oil (the cheapest) that I
have found to work very well: it was still liquid five years after I applied it to numerous
clocks. Two of my suppliers told me that it is not a clock oil (even though it said �clock
oil� on the bottle) but rather a light machine oil: in other words, a highly refined mineral
oil, similar to kerosene in appearance, consistency and smell. The only problem I have
experienced with this oil is that it is too thin (at room temperature, here in Texas) and
runs too easily when applied to clock bushings (but it has worked very well on my pocket
watches). I have had disappointing experiences with three synthetic lubricants and
therefore do not use them.
Now for the disclaimer to keep me out of trouble:
1. To lubricate a clock, use only an oil that says �clock oil� on the bottle.
2. To lubricate an electric clock motor, use only an oil that says �oil for electric motors�
on the bottle.
3. To lubricate a watch, use only an oil that says �watch oil� on the bottle.
4. To lubricate your car, use only an oil that says �car oil� on the bottle.
5. Experiment at your own risk!
Clock Repair Main Page
Escapements in Motion
Links Page
Tributes Page
Home Page