|
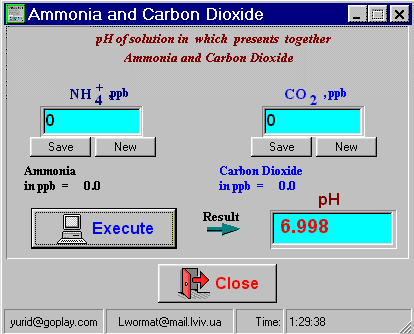
Figure 1. Evaluate pH range from data of NH3 and CO2 . Download134 kb At this calculation shown very simple, but more than important fact about small influence on the pH value of Dioxide Oxygen (*normal* pH value NOT GUARANTED small concentration of CO2). |
|---|
|
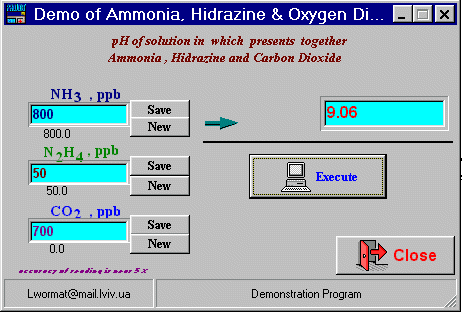
Figure 2. Evaluate pH range from data of NH3, N2H4 and CO2 . Download (132 kb)One different is at here - 3 parameter to calculation. Main problems to use chemical recalculation are:
|
|
|---|
There is to shown short issue about relation between pH and Conductivity of diapason.
On example of recalculation of the Drum Boiler Water is demonstrate it's relation.
If You have possibility to check Log-Sheets-Books at the Chemical Department by the Power Plant - it's to found many mistakes and elementary incomprehension so simple and important data of. I'd to check graduate specialists from reputable internationally known of Consulting/Insurance/Engineering Companies who don't to find nothing special in a some of absurd and ridiculous of lab data.
|
|
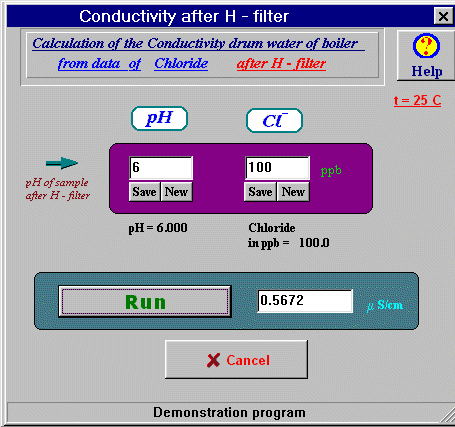
Picture 1. The Limitation between pH and Conductivity range from data of The Boiler Drum's Water . Download222 kb In this calculation is displyed why, for example is so funny pH less than 6 to found in drum boiler water when Conductivity in 0.3-0.9 mkCm/cm. Check on the picture - also is so many of possibilities to print the same absurd results, likes pH=7.5 K=0.95 mkCm/cm... |
|---|
|
Figure 3. Evaluate Conductivity,pH and Chloride range from data of the Drum of Boiler Water. Download (222 kb)This demo give possibility to fast and enough accurate to evaluate Chloride concentrations - what's hard or impossible to another methods using in the practice day-to-day exploitation... |
|
|---|
At the next time we would publish demo of more associated to the volatile method of boiler watertreatment and to phosphate too. We are still looking for co-operate in developing of software to the Chemical Monitoring and Manage of Main processes at the Power Plant to the prevent problems of the corrosion damages and lost effectively and safety of exploitation due to of deposits presence.
More details for request.
back to previous page