On earth, there are a very few elements which can be used as sources of power, and even fewer of these elements can be harnessed, and their power used for productive purposes. By far the most abundant and most important of these elements is Uranium. In this paper, we will examine the chemical processes involved in each of the stages which bring Uranium to the state in which it is used in Nuclear Reactors, and the disposal of wastes after use.
Cosmologists speculate that approximately 65 billion years ago, a star system nearby to our solar system went
supernova, emitting a blinding light which was 108 times as bright as the light of our sun. The incredible amount of heat released during this process allowed for the fusion of elements to occur. When the star went supernova, huge quantities of neutrons burst out in all directions. Since there is no electrical repulsion between a neutron and the nucleus of an atom, the neutrons quickly added themselves to the nuclei of the elements which were already present (primarily Hydrogen and Helium). The high buildup of neutrons inside of these Hydrogen and Helium atoms resulted in beta decay, changing the neutrons into protons, transmuting the large, unstable nuclei of the atoms into more stable forms, which represent all of our elements above atomic number 26 (Iron). These new elements formed meteors and were expelled from the supernova with such force that they eventually ended up here, in our solar system.
(internet - 1)
This is how we account for all of our larger and fissionable elements, the most useful being Uranium. Uranium has atomic number 92, with 92 protons and electrons. There are several isotopes of Uranium which can occur, but by far the two most common isotopes are U-238 and U-235. U-238 is more common than Tin, and is responsible for the movements of tectonic plates. U-235 is the fissile isotope of Uranium. The relative occurence of the two isotopes is such that 99.2 % of all Uranium is of the U-238 isotope, whereas only 0.72 % of Uranium is of the U-235 isotope.
(internet - 2)
Uranium can be found almost anywhere in the world. It exists in the ocean mantle as well as the continental crust, and even in sea water. The occurences of Uranium in the ocean mantle and sea water are relatively small, ranging from 0.003 ppm up to 4 ppm, incorporating many secondary minerals. The main source of the world's Uranium comes from ore deposits in the continental crust. Internet - 3 Uranium ores generally have a less than 1 % concentration of Uranium, but the richest Uranium ores in the world, which are all in Saskatchewan, Canada, can have up to 20 % concentration.
(Internet - 4)
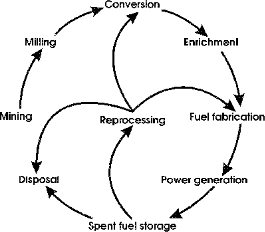
The cycle associated with Uranium is a complex one. Many stages are involved, and many steps within each stage. It follows roughly the following pattern :

Because it does not naturally occur in pure form, the processes of mining and milling Uranium becomes more complex. There are several proven ways to mine Uranium. Open-pit mining and underground mining are fairly self-explanatory, and they are fairly minor in practice. By far the most common form of Uranium mining is a process called In Situ Leach mining.
In Situ Leach mining involves the use of chemicals to dissolve the Uranium ore in its place, so it can later be recovered without harm to the ground where the Uranium ore lay. It is a technique in which sulphuric acid is leached into the Uranium deposits (UO3), where the following reactions occur:
First, the UO3 is converted into UO22+:
Then the Sulphuric acid is added:
The first step in the milling process is to purify and concentrate the Uranium, making the rest of the milling process more more efficient. In order to achieve this, one of two methods can be used. The first, ion exchange, involves using other chemicals of the same electrical charge to replace undesirable elements within the Uranium compound. The second, and more effective method of purification, solvent extraction, is a continuous process using tertiary amines to react with the Sulphuric acid , yielding an organic compound, as demonstrated by the following reaction:
The Uranyl sulphate then reacts with the Amine sulphate ((R3NH)2SO4(org)), drawing the Uranium into the organic compound. Meanwhile, the unwanted sulphate ions are left in the aqueous form:
After removing the solvents which are no longer needed, ammonia is added in order to neutralize the solution, and a precipitate of ammonium diuranate ((NH4)2U2O7) is formed. The ammonium diuranate is then dried and heated, which yields a solid, purified Uranium oxide (U3O8). This form of Uranium is commonly referred to as Yellowcake because of its pale yellow colour. Yellowcake usually consists of 70 % to 90 % Uranium. Once the Yellowcake has been formed, the Uranium milling process is complete. The Yellowcake is shipped to a refinery for further processing.
(internet - 2)
Naturally occuring Uranium has approximately 0.72 % of the fissile U-235 isotope, as earlier stated. This presents a problem, because in order for Uranium to be useful in terms of use in nuclear reactors, it must be composed of at least 3 % U-235. This requires the Uranium to be enriched with the U-235 isotope. The most commonly used method of Uranium enrichment is gaseous diffusion. This process requires Uranium hexafluoride (UF6). The purpose of the refining and conversion stages in the nuclear cycle is to convert the Uranium oxide concentrate into Uranium hexafluoride, in preparation for the enrichment process.
(literary - 1)
First, the yellowcake is dissolved in nitric acid, which results in the formation of Uranium nitrate:
The Uranium nitrate is then exposed to a continuous solvent extraction process. Tributyl phosphate is used to extract the uranium into an organic phase (kerosene), again leaving the unwanted substances in the aqueous state. Dilute nitric acid is then used to wash the purified Uranium out of the kerosene. After concentration by evaporation, the product is pure Uranium nitrate
(UO2(NO3)2). When heated, UO3 is produced in its pure form.
(internet - 2)
The first stage in the process of conversion into UF6 is to reduce the Uranium oxide with Hydrogen in a kiln:
The Uranium oxide is then pumped into another kiln, where it is combined with gaseous Hydrogen fluoride to form Uranium tetrafluoride:
Gaseous Fluorine is then fed through the Uranium tetrafluoride to obtain Uranium hexafluoride:
Once the conversion to Uranium hexafluoride is complete, the process of enrichment by gaseous diffusion can begin. Since Uranium hexafluoride has a boiling point of approximately 65°C, gaseous diffusion can take place at relatively low temperatures, making it more economically efficient for large-scale production than other methods.
Internet - 5
Gaseous diffusion is performed by passing the Uranium hexafluoride, containing both U-235 and U-238 isotopes, through a narrow passage lined with a barrier which has billions of pores per square centimetre:
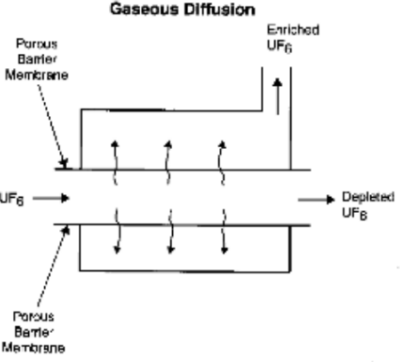
Because U-235 has fewer neutrons in its nucleus than U-238, it also has a lower atomic mass. Because of its lower mass, U-235 moves slightly faster through the gaseous diffusion passage than the other isotope. More U-235 atoms diffuse through the porous barrier than U-238 atoms. Therefore, the gas collected on the far side of the barrier is slightly enriched with the U-235 isotope. The enriched UF6 is removed from one end of the diffuser reprocessed in a cascade of other diffusers which repeat the process, and the depleted UF6 is removed from the other end. The degree of enrichment obtained from a single diffusion is negligible, and the process must be repeated up to 4000 times in order to obtain the 3% - 5% enrichment required by nuclear reactors.
(Internet - 4)
The gaseous diffusion method of Uranium enrichment accounts for over 90 % of the world's enriched Uranium. There are, however, other methods of Uranium enrichment. Two such methods are centrifuge enrichment and laser enrichment. Centrifuge enrichment is a superior method, in that it requires less energy than gaseous diffusion to perform, and it yields a higher concentration of the U-235 isotope. It is used primarily in the formation of weapons grade Uranium, which requires a much higher enrichment of U-235 than nuclear reactors do. It is not in common practice around the world, however, due to the fact that it produces enriched Uranium on a much smaller scale, and is more expensive than gaseous diffusion.
(Internet - 4)
Centrifuge enrichment also makes use of the small mass difference between the U-235 and U-238 isotopes of Uranium. It involves placing UF6 in a powerful turbine and spinning it at 50 000 to 70 000 rpm. At this speed, the heavier atoms increase in concentration along the outside of the cylinder, wheras the lighter ones congregate around the centre of the tube. The enriched gas can then be separated from the depleted gas and the process is repeated. Wheras gaseous diffusion requires thousands of repetitions of the process, centrifuge enrichment can obtain a satisfactory enrichment level after 10 or 20 repetitions.
(Internet - 4)
Companies in various countries around the world have been researching a new form of Uranium enrichment using lasers tuned to specific wavelengths, which would ionize one of the Uranium isotopes. The isotopes could then be separated with the use of magnetic fields.
(Internet - 4)
Once the proper enrichment process has taken place, UF6 is converted into UO2, and then formed into fuel rods for use in nuclear reactors. Inside the nuclear reactor, the Uranium undergoes a fission reaction. This reaction occurs when a single neutron is injected into the nucleus of a Uranium atom. The addition of the neutron makes the nucleus of the Uranium atom unstable, and the result is the induced fission of the atom:
Each of the three neutrons released in the nuclear reaction collide with a new Uranium atom, producing another reaction, and producing another three neutrons, and the chain reaction continues. By harnessing the energy released by each individual reaction, massive amounts of energy can be obtained in a very little amount of time.

A typical Uranium atom produces 3.2 x 10-11 Joules during fission. This is significantly higher than the 6.5 x 10-19 Joules for the combustion of Carbon. This demonstrates the vast difference between the use of conventional fuels and the use of nuclear fission as fuel. Besides Barium and Krypton (indicated above) there are several possible fission products for Uranium, such as Strontium, Cesium, Iodine and Xenon. Some of these fission products are fissionable themselves, and decay further until a stable isotope is reached. The fission of Uranium follows a specific pattern.
Fission inside a nuclear reactor is very volatile, and precautions must be taken in order to prevent accidents from occuring, and to prevent the reaction from getting out of control. Since the main cause of the nuclear reaction is the neutrons being expelled during fission, safeguards are placed inside the reactor, such as control rods of Lithium and Boron, which act as neutron absorbers, keeping the reaction under control.
(Internet - 5)
Once the Uranium fuel has been used in a nuclear reactor, there are environmental issues which arise. Because of the radioactive nature of depleted Uranium, storing it can result in damage to the surrounding biological compounds and to water supplies in the vicinity of the radioactive waste.
(Internet - 4)
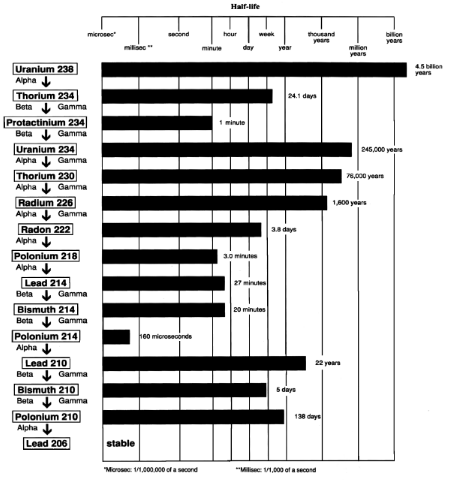
Uranium which has been depleted of the U-235 isotope can have many different roles in the industrial world. Due to its high density, it is very heavy, making it suited for use as airplane counterweights, or yacht keels. Its most prominent use is as radiation shielding, where it is five times more effective than Lead. This is possible due to the long half-life (4.5 billion years), and therefore low radioactive emissions.
(Internet - 4)
The spent fuel, after being chopped up, is dissolved in hot concentrated nitric acid. A countercurrent solvent extraction process is used to separate the Uranium and Plutonium from the rest of the fission products. Tributyl phosphate is dissolved in either kerosene or dodecane, and used to draw the Uranium and Plutonium into the organic phase while leaving the other fission products as aqueous solutions. Plutonium is then transferred back to the organic phase, where it is washed out with dilute nitric acid and stripped of the organic solution, forming Plutonium nitrate. Both the Uranium and the Plutonium are then concentrated by evaporation to form PuO2 and UO3. Just as in the milling process, the Uranium is reduced using Hydrogen to form UO2.
(Internet - 4)
The Uranium is now at a critical point in its cycle. It can either be reprocessed, by going through the conversion and enrichment processes again, and be used for fission inside a nuclear reactor for a second time, be used for other purposes in the industrial world, or it can be sealed in drums and buried to await disposal underground or in specially designed above-ground sites.
(internet - 2)
By examining all of the chemical processes involved in the various stages in the creation, production and use of Uranium, we have demonstrated how useful Uranium is to us and our society, with regards to energy production. This examination has shown the unique nature of Uranium and why it is the most important of all fissionable elements.
Well, that's it for our magical journey through the funky and fantabulous world of Uranium. Thank you all for visiting my super duper splendiferous web site. Come back soon! Tell your friends!
By the way ...
Due to the overwhelming amount of information related to this topic, I was unable to go in depth into every aspect of Uranium's Chemical cycle. If you're interested in learning more about Uranium than what is located in my project, please feel free to visit any of the excellent web sites located in my Bibliography. These were my main resource while doing my Independent Study. They are all excellent sources of information, and have my full personal endorsement.