

A lunar landscape
The remains of a burning floor at Sandsend Alum works, over 100 years after closure.

The cliff profile
Whitby Mudstone Formation from the foreshore north of Sandsend
At Saltwick bay south of Whitby a huge ampitheatre has been carved out of the back wall of the bay and Saltwick nab is still stained red from the dumps of burned shale. Massive workings are also visible at Kettleness at the eastern end of Runswick Bay. At Ravenscar the alum workings just inland have been preserved as a geological trail.
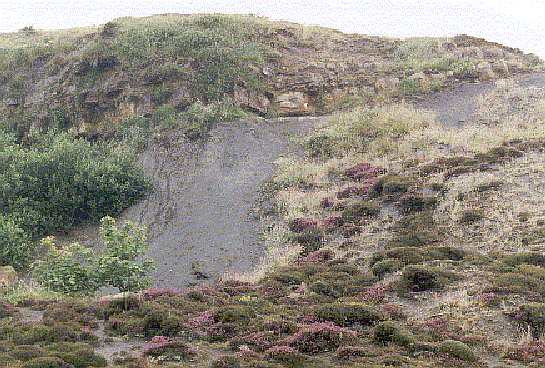
Dogger formation overlying Alum Shales
Sandsend Alum Quarries
Approximately 15 meters of grey pyritic shale make up the Main Alum Shales. This is
underlain by a thin band of sideritic mudstone and the Basal 6 meters is an almost
non-bituminous shale known as the Hard Shale. Large numbers of the ammonites
Hildoceras Bifrons and Dactylioceras Bifrons can be found in the alum shales as
well as the small bivalve Dacryomya (formerly Nunculana) Ovum.It was the presence ofthis fossil that told the quarrymen that the shale was suitable for alum making, and it is now known that this shale is low in CaCO3 which would neutralise the sulphuric acid produced by the roasting of the pyrites.
Icthyosaur and Plesiosaur remains were formerly plentiful and occasional good specimens, some articulated are still to be found. So common were reptillian remains in former days that Fox-Strangeways and Barrow noted in 1915 that large vertebrae could be found bordering garden paths around Whitby. The great crocodile Stenosaurus and the sturgeon like fish Gyrosteus mirabelis have also been found.
The ammonite Hildoceras is named for the Abbess of Whitby St. Hild or Hilda (614-680). (external link) The legend recorded by Scott in Marmion is that ammonites were snakes turned into stone by St. Hilda. Enterprsing locals gave credence to the legend by carving snakes heads onto ammonites! The arms of Whitby incorperate three 'snakestones'.
Alum is the double sulphate of Aluminium and either Potassium or Ammonia. It was of
great importance in tanning and in the dying industry where it was and still is used
as a mordant (fixative).
Before the fifteenth century its manufacture was a secret of the Arabs and the Turks.
In the fifteenth century alum rocks were discovered near Rome and in the Christian
world Alum making was a Papal monopoly. In 1607 alum shale were discovered in
Britain at Belman Bank, Guisborough in Cleveland and alum manufacturing began there
as a Royal monopoly. In 1649 the monopoly was ended and manufacturing began in the
many areas that alum bearing rocks had been found. In the mid-nineteenth century it
was discovered that alum could be extracted from shales produced as colliery waste
and the alum shale quarrying industry collapsed.
The most successfully quarry was the one at Lythe near Sandsend working from 1615 to around 1870. (Raistick 1965 gives a date of 1867, Scarborough Borough Council Community Programme Agency, undated give 1871)
The shale was quarried in vast amounts and calcined by burning enormous heaps up to
15 meters high and 30 meters across. The heaps were piled up over brushwood or
gorse and set alight. Due to the oil content of the shales these piles could burn
for up to a year. The burnt shale was then steeped in water to extract the sulphates
of iron and alumina formed by the action of sulphuric acid, derived from the pyrites.
The resulting liquor was sent to the alum works, usually close by. There it was
boiled and an alkali added, this was either potash derived from burnt seaweed
(potassium source) or urine (ammonia source). The urine was collected locally from
jars people left on their doorsteps but this supply was inadequate. Urine was
imported from London and Hull, and the first public urinals in Hull were built as
collecting points for this trade !
Wood was initially used as fuel but the industry exhausted local supplies of wood
as it grew and by 1700 10,000 tons (imperial) of coal were being brought from
Sunderland and Tyneside to the Yorkshire alum works. By 1805 this has doubled to
20,000 tons of coal.
As the liquor evaporated the alum crystalized before the iron salts which could be pumped off. Judging the point at which the maximum amount of alum had been extracted while the iron salts were still in solution was the alum makers great secret. This wonder of craft technology was carried out by floating an egg in the liquor as a crude hydrometer.
back to the mining links page
Return to the East Yorkshire Coast Geology and Geomorphology page