Chemical Constituents of life (part 1)
The chief organic components in cells are:
a. carbohydrates
b. lipids
c. proteins
d. nucleic acids
carbohydrates, proteins and nucleic acids are called polymers which are giant molecules made from many repeating units called monomers
A. Carbohydrates
- they are substances with general formula Cx(H20)y,where x and y are variables
- all carbohydrates are aldehydes or ketones and all contain several hydroxyl group
- it is divided into 3 main classes: monosaccharides, disaccharides, polysaccharides
Major role played by carbohydrates in cells
- simple carbohydrates are the principal energy source in the cell
- long-chain carbohydrates act as food storage compounds e.g. starch, glycogen
- long-chain carbohydrates form some of the structural components e.g.cellulose
- structural components of
1. membranes
2. nucleic acid
3. ATP
Monosaccharide
- general formula: (CH20)x, x=3-9
- They are either aldose or ketose
Examples of monosaccharides:
1 . Triose(3 carbon sugar)
- intermediate products along the photosynthetic and respiratory pathways.
Usually as triose phosphate e.g.glyceraldehyde
2. pentose(5-C sugar)
- ribose- constituents of nucleic acid
- component of ATP, nucleotide coenzyme, e.g. NAD, FAD
- ribulose- carbon dioxide acceptor(ribulose bisphosphate)
3. hexose(6 -C sugar)
- glucose- respiratory substrate
build up other disaccharides and polysaccharides
- fructose - respiratory substrate
constituents of nectar and honey
build up polysaccharides
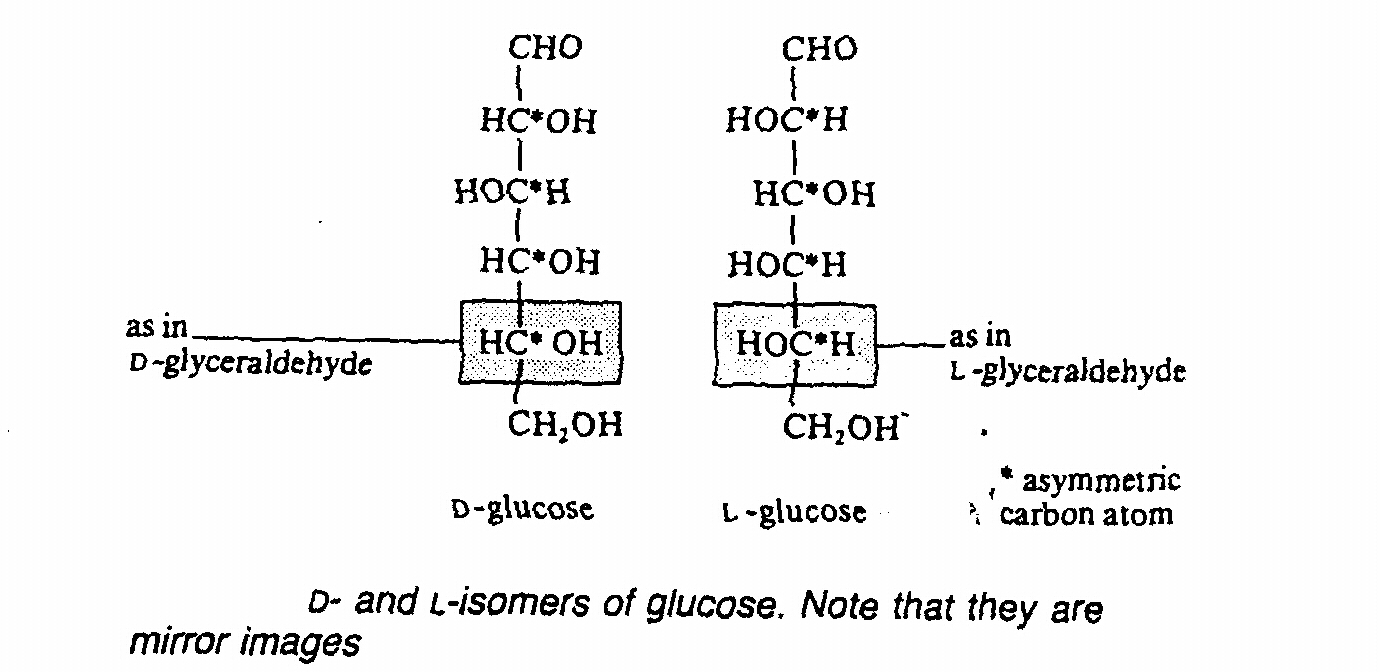
All monosaccharides exist 2 forms: D(+) or L(-) form due to different spacial arrangement of molecules(stereo- isomers)

- all natural occurring monosaccharides are D-isomers
- both D and L isomers have same physical and chemical properties but different 3-d structure which have
significance in enzymatic reaction
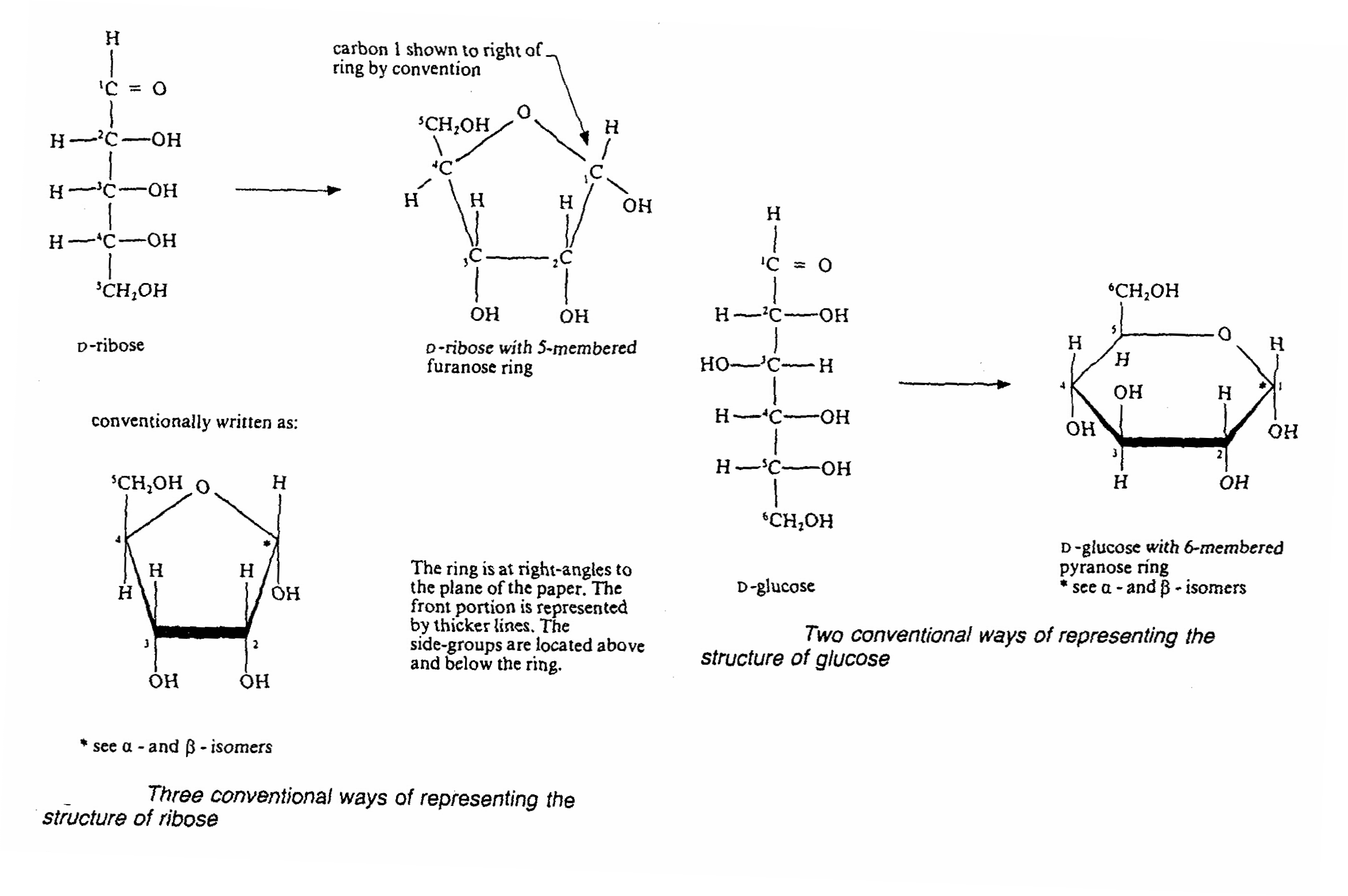
- 5-carbon and 6-carbon sugar usually exist in ring form and it is usually in this form the sugar incorporated into
disaccharides and polysaccharides
- due to the ring structure, another type of isomer is formed: αisomer and β-isomer
Disaccharides
- with the general formula of C12H22011
- some are reducing while some are non-reducing
- they are formed by condensation reactions( involved the loss of a single water molecule)
between two monosacharides usually hexoses. This process can be repeated indefinitely
to build up the giant molecule of polysaccharides
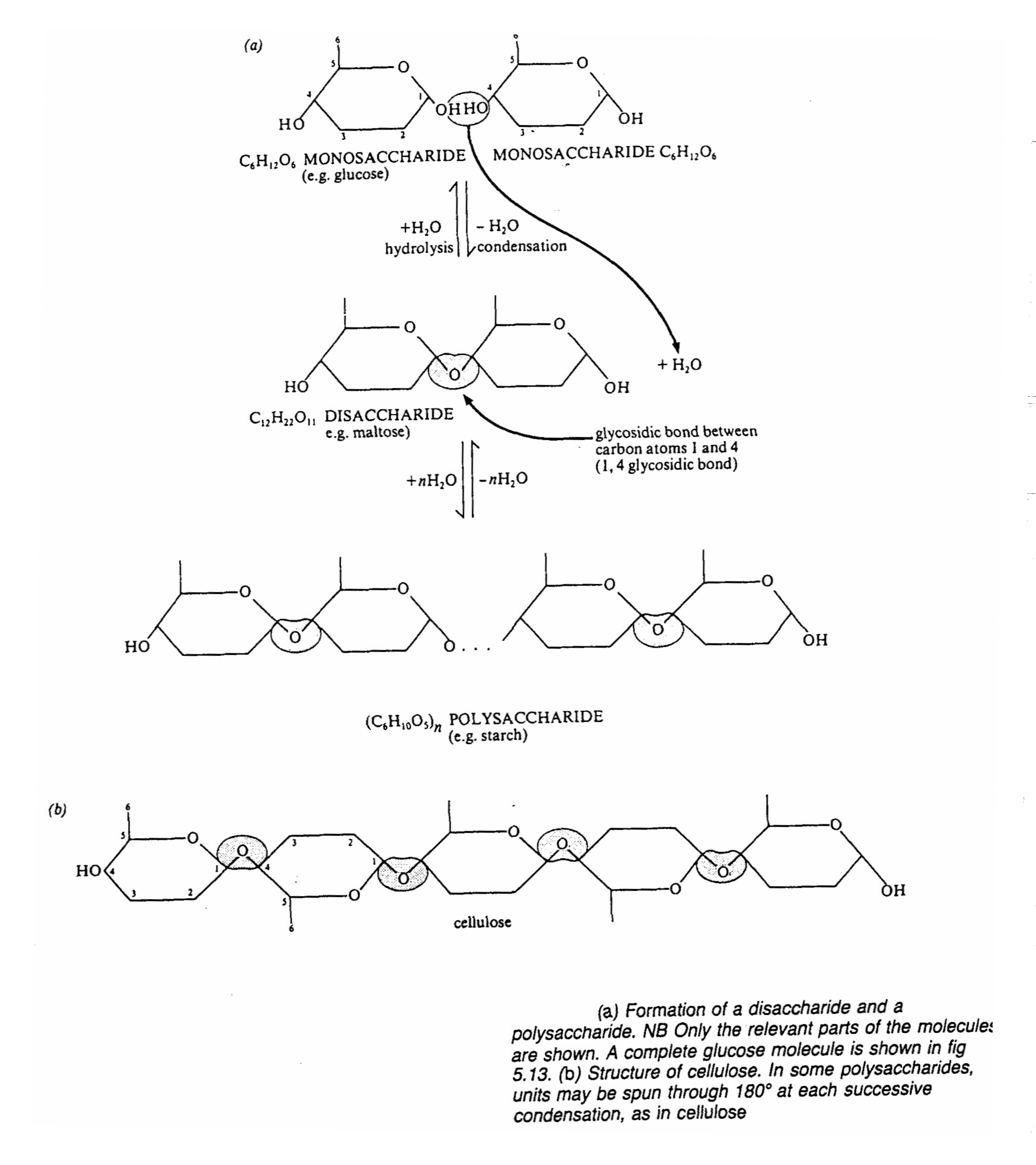
- the bond formed between two monosaccharides is called a 1,4-glycosidic bond. The monosaccharide units are
called residue once they are linked
- The most common disaccharides are maltose, lactose and sucrose
- Maltose is made up of two glucose molecules, it is a reducing sugar
- Lactose (milk sugar) is made up of glucose and galactose. It is found in milk and is a reducing sugar
- Sucrose (cane sugar) is made up of glucose and fructose. It is found in large quantities in the phloem of plants. It
can be obtained from sugar cane i.e. it is the sugar we normally buy from shops. It is a non-reducing sugar.
Polysaccharides
- it is made up of many monosaccharides molecules through the process of condensation
- their main function is as food and energy stores(starch and glycogen) and as structural materials.
They are convenient storage molecules because:
1. Their large size makes them more or less insoluble in water, so they exist no osmotic and chemical influence
in the cell;
2. They fold into compact shapes and can easily be converted to sugar by hydrolysis when required.
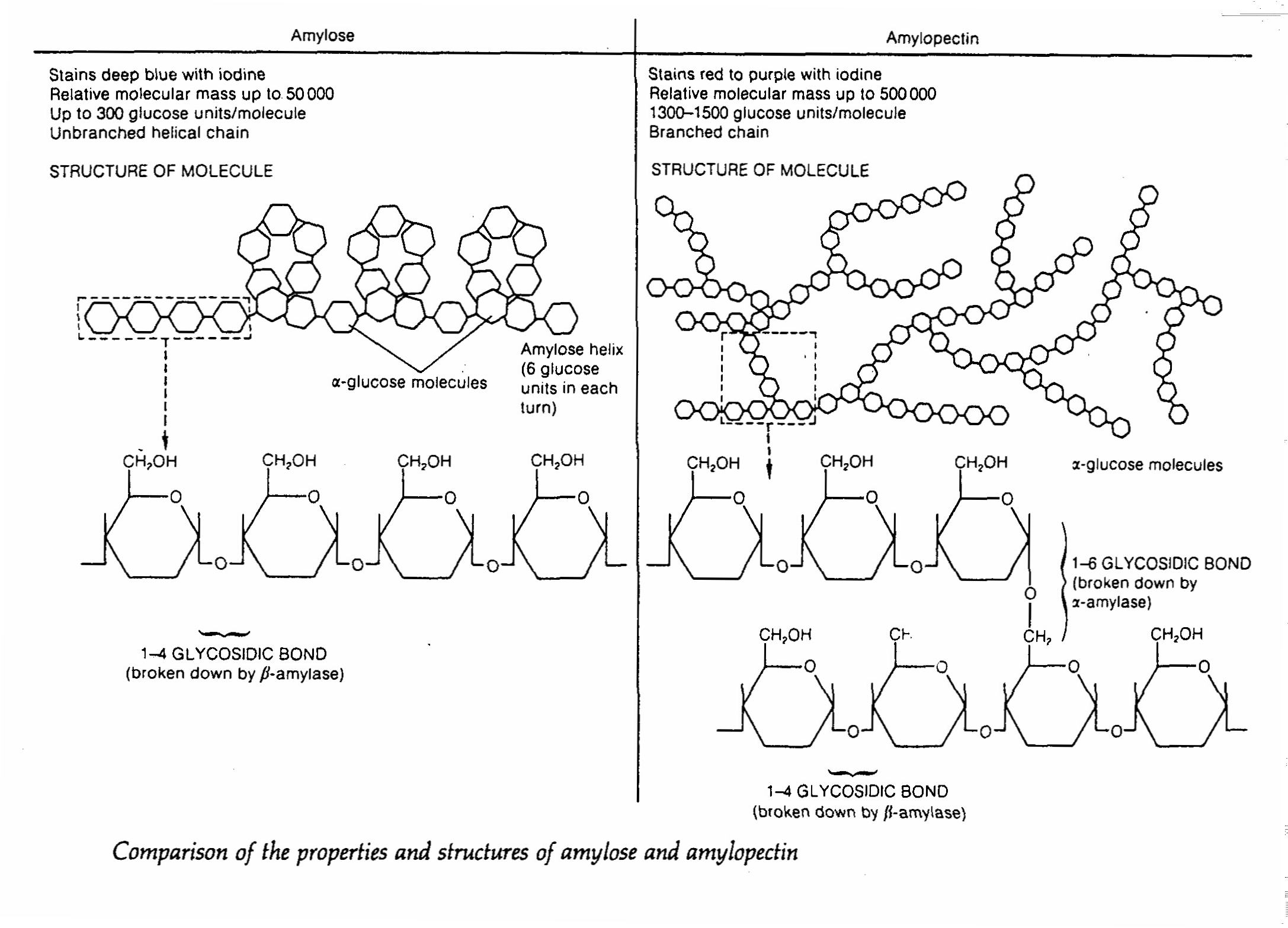
1. Starch
- it is a polymer of glucose
- it acts as the main food store in plants which is absent in animals
- it is a mixture of amylose and amylopectin
- amylose has a straight chain structure consisting of several thousand glucose residues, the chain coils helically
into a more compact shape
- amylopectin has twice s many glucose residues as amylose and it has many branches formed by 1,6- glycosidic
bonds.
- It is store mainly in liver and muscles - Like starch, it is made up of α-glucose molecules and exists as granules - It is similar to amylopectin in structure but it has shorter chains and is more highly branched2. Glycogen
It is the major polysaccharides storage material in animals and fungi
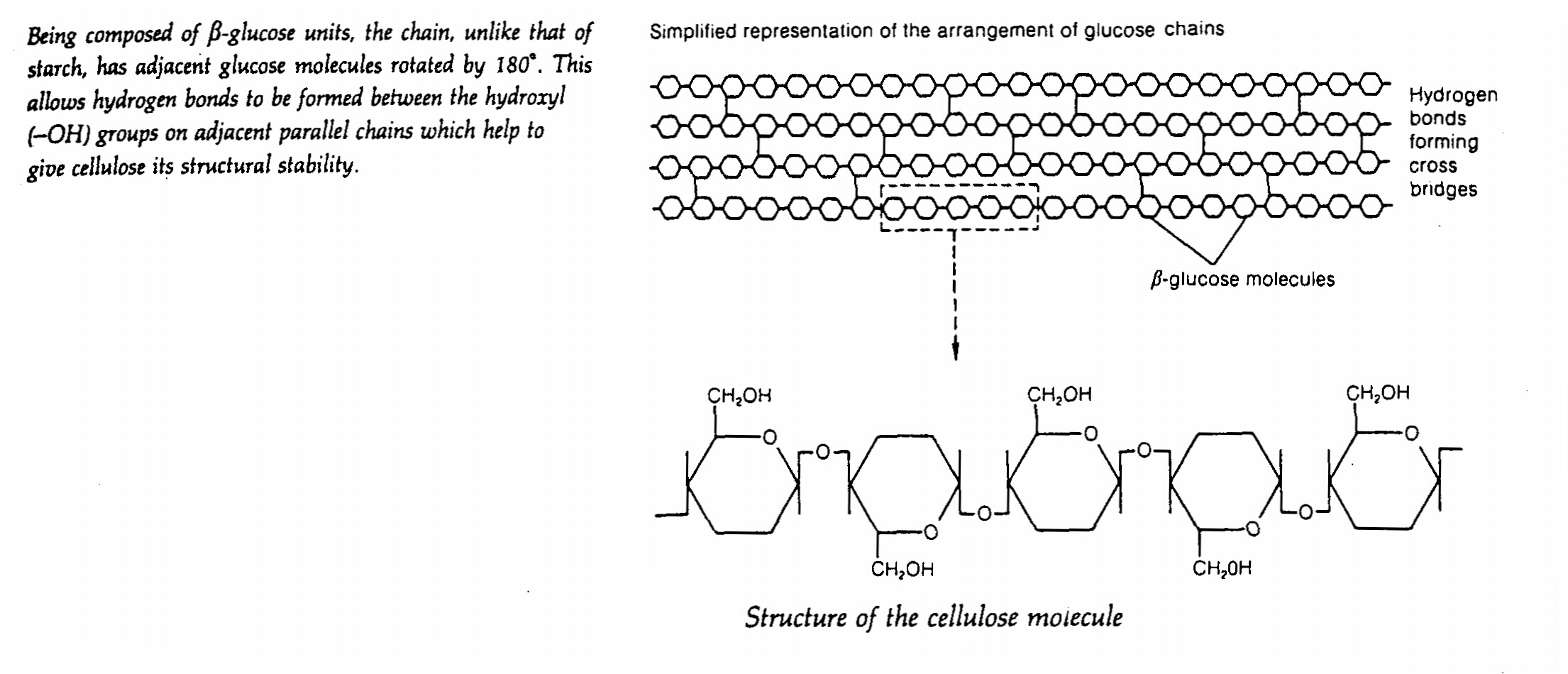
3. Cellulose
- it typically comprises up to 50% of a plant cell wall
- it is a polymer of around 10000 β- glucose molecules forming a long unbranched chain. Many chains run parallel to
each other and have cross linkages between them. The chains associate in groups to form microfibrils which are arranged
in larger bundles to form macrofibrils. These help to give cellulose its considerably stability which makes it a valuable
structural material
- apart from being a structural material, cellulose is an important source of food for some animal, bacteria and fungi
- commercially, cellulose is used for making cotton goods and act as a constituent of paper
NB:Throughout the following tests, the Benedict's reagent can be substituted with Fehling's solution.
It does, however, require the mixing of two Fehling's solution A and B immediately prior to the tests.
Test for reducing sugar
Precautions
Once the water bath has reached boiling point, the source of heat should be turned down.
Reactions will still take place even if the temperature is slightly below 100℃
Method
1 . Bring the water in the water bath up to boiling point and turn down the source of heat.
2. Take 2cm3 of the solution to be tested and add 2cm3 of Benedict's reagent. Mix the reagents thoroughly
3. Place the test tube in the water bath and leave for 5 minutes, shake occasionally
Results
When a reducing sugar is present , it will reduce soluble copper (II) sulphate to insoluble copper(I) oxide which forms a precipitate.
In addition, the blue copper (II) sulphate become brick-red colour copper (I) oxide. The more reducing-sugar , the greater the
amount and darker the colour of the precipitate. The colour of precipitate will change from blue to green, then to yellow and then
through orange and brown to deep red as the quantity of reducing sugar increases.
Test for non-reducing sugar
There is no specific test for a non-reducing sugar as such, but a non-reducing sugar can be detected by its inability to reduce
Benedict's reagent directly. If it is then hydrolysed by boiling with dilute hydrochloric acid it will be broken down into its
constituent monosaccharides. These will then reduce Benedict's reagent in the normal way. A non-reducing sugar is thus identified
by a negative reaction to Benedict's reagent before hydrolysis and a positive result after hydrolysis
Method
1. Carry out reducing sugar test(as stated before)
2. Add I cm3 of dilute hydrochloric acid to a fresh sample of 2cm3 of the solution to be tested, mix the solution
and boil 2-3 minutes
3. Add sodium hydrogen carbonate solution until the solution is neutral or preferably slightly alkaline. Use pH
paper to test for this. ( This procedure is necessary because Benedict's reagent is not effective in acid conditions)
4. Carry out the reducing sugar test again.
Results
A negative result(solution remain blue) after the first reducing sugar test, followed by a positive result(solution turns red/brown)
after the second reducing sugar test is an indication of a non-reducing sugar
Detecting the presence of a non-reducing sugar in a solution which also contains reducing sugar
As boiling with hydrochloric acid does not affect a reducing sugar, its presence means that a positive result will always be obtained
when carrying out a non-reducing sugar test. It may, however, still be possible to detect a non-reducing sugar in the presence of a
reducing sugar; much depends on the relative concentrations of each. If the amount of reducing sugar is small, detection of
non-reducing sugar should be possible.
Method
1 . Add 2cm3 of the solution under test to each of two test tubes
2. Carry out the reducing sugar test on one solution and the non-reducing sugar test on the other.(NB It is most important that the
quantities of Benedict's reagent used in each case are exactly equal and that both are boiled for the same length of time)
3. Compare the colour and amount of precipitate in each of the tubes
The amount of precipitate should be greater and the colour darker on carrying out the non-reducing sugar test. If one considers a
solution containing equal concentrations of glucose(reducing sugar) and sucrose(non-reducing sugar), the glucose alone will reduce
Benedict's reagent when boiled with it. Upon hydrolysis by hydrochloric acid, the sucrose will be converted to glucose and fructose,
both reducing sugars. The amount of reducing sugar is now greater and so boiling this solution with Benedict's reagent produces
more reduction. This second solution should therefore be brick red in colour and contain a greater quantity of precipiate.
Test for starch
Method
1 . Place two drops of the solution to be tested in a depression in a spotting tile, or in a test tube.
2. Add a drop of iodine in potassium iodide solution
Results
If starch is present, the yellow-orange iodine in potassium iodide solution becomes a blue-black colour. The iodine takes up a
position in the centre of the starch helix, forming a starch -iodine complex and giving rise to the intense blue-black coloration.
B. Lipids- contain carbon, hydrogen and oxygen
- insoluble in water but can dissolve in organic solvent such as acetone, alcohol and others
- they are esters of fatty acids and an alcohol
- the ester linkage is formed by a condensation process
Fatty acid
- contain the acidic group -COOH, with general formula of R-COOH where R is hydrogen or an alkyl group. The
number of carbon of fatty acids ranges from 14 to 22. The long chain of carbon and hydrogen form a hydrogen
tail. This tail is hydrophobic(water hating), so lipids are insoluble in water
- lipids containing fatty acids with double bonds are called unsaturated lipids while those lacking double bonds are called
saturated lipids
- lipids are classified into oils and fats. Oils are liquids at room temperature while fats are solids at room
temperature(20 ℃) Oils contain larger proportion of unsaturated lipids while fats contain larger proportion of
saturated lipids
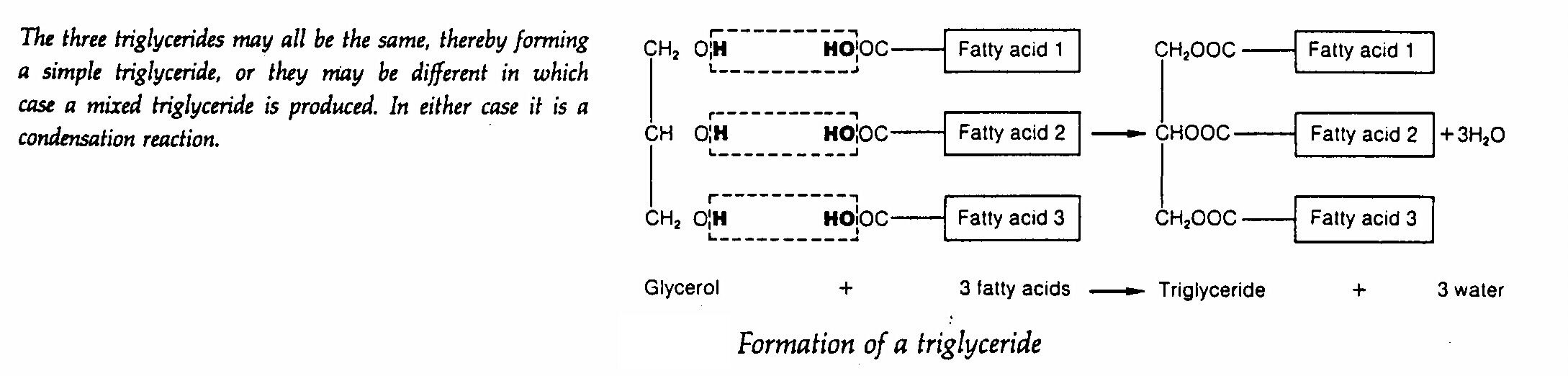
Glycerol
- most lipids are esters of alcohol glycerol and so lipids are therefore called glycerides
- the three hydroxyl groups of glycerol condense with three fatty acids and the lipids formed are called
triglycerides
1 . as energy source- they have a higher calorific value than carbohydrates
2. storage- they are excellent energy stores. For the same amount of energy stored they possess less than half the mass of
carbohydrates. This makes them especially useful for animals where locomotion requires mass to be kept to a minimum. In plants
they are useful in seeds where dispersal by wind or insects makes small mass a necessity. Their insolubility is another advantage,
as they are not easily dissolved out of cell
3. Insulation- Fats conducts heat slowly and so are useful insulators. In endothermic animals, such as mammals, it is stored beneath
the skin(subcutaneous fat) where it helps to retain body temperature. In aquatic mammals, they have extremely thick subcutaneous
fat, called blubber, which forms an effective insulator
4. Protection- act as shock absorber around internal organs
Phospholipid
- they are lipids containing a phosphate group i.e. one fatty acid is replaced by phosphoric acid
- phosphoric acid is hydrophilic(water loving) while the remainder of the molecule is hydrophobic
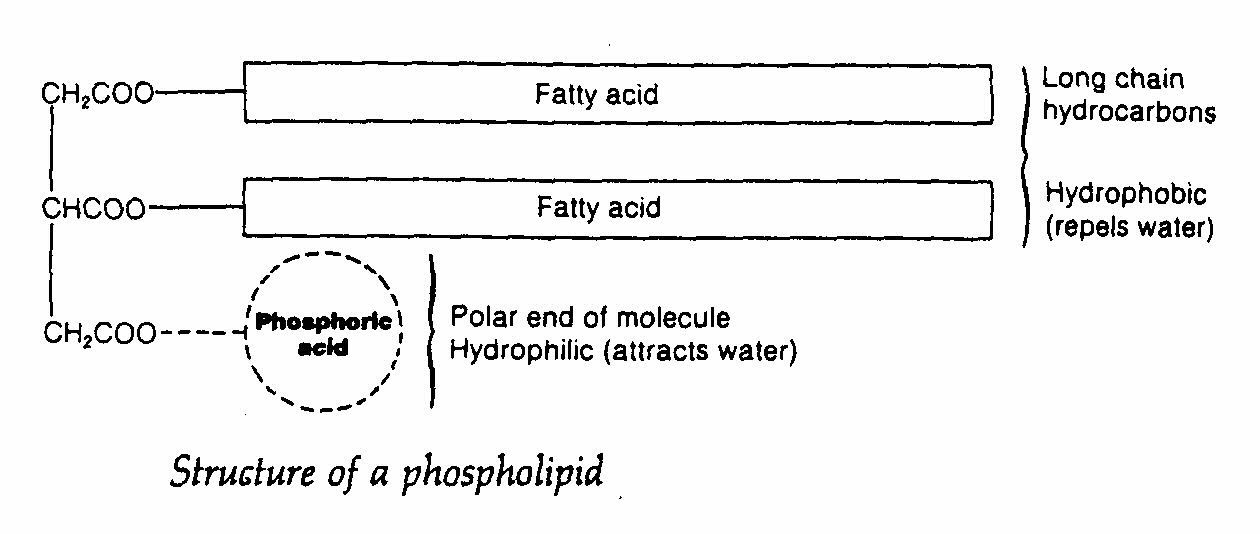
Functions of phospholipid
- it is the major component of plasma membrane