Nucleic acids
- like protens, they are essential for life, they constitute te genetic material of
all living organisms
- there are 2 types of nucleic acids: deoxyribose nucleic acid (DNA) and ribose nucleic
acid (RNA)
- they contain the information which control the activities of cells and organisms
- they are made up of units called nucleotide, they will arrange to form extremely long
molecules
known as polynucleotides
Nucleotide
- a nueleotide has 3 components: 5-C sugar, a nitrogenous base and phosphoric acid
1 Sugar
- it has 5 carbon atoms i.e. it is a pentose
- there are 2 types of nucleic acid depending on the pentose they contain. Those with
ribose are called ribonucleic acid(RNA) and
for those which contain dexoyribose(ribose
with oxygen removed from carbon atom 2) is
called deoxyribose nucleic acid(DNA)
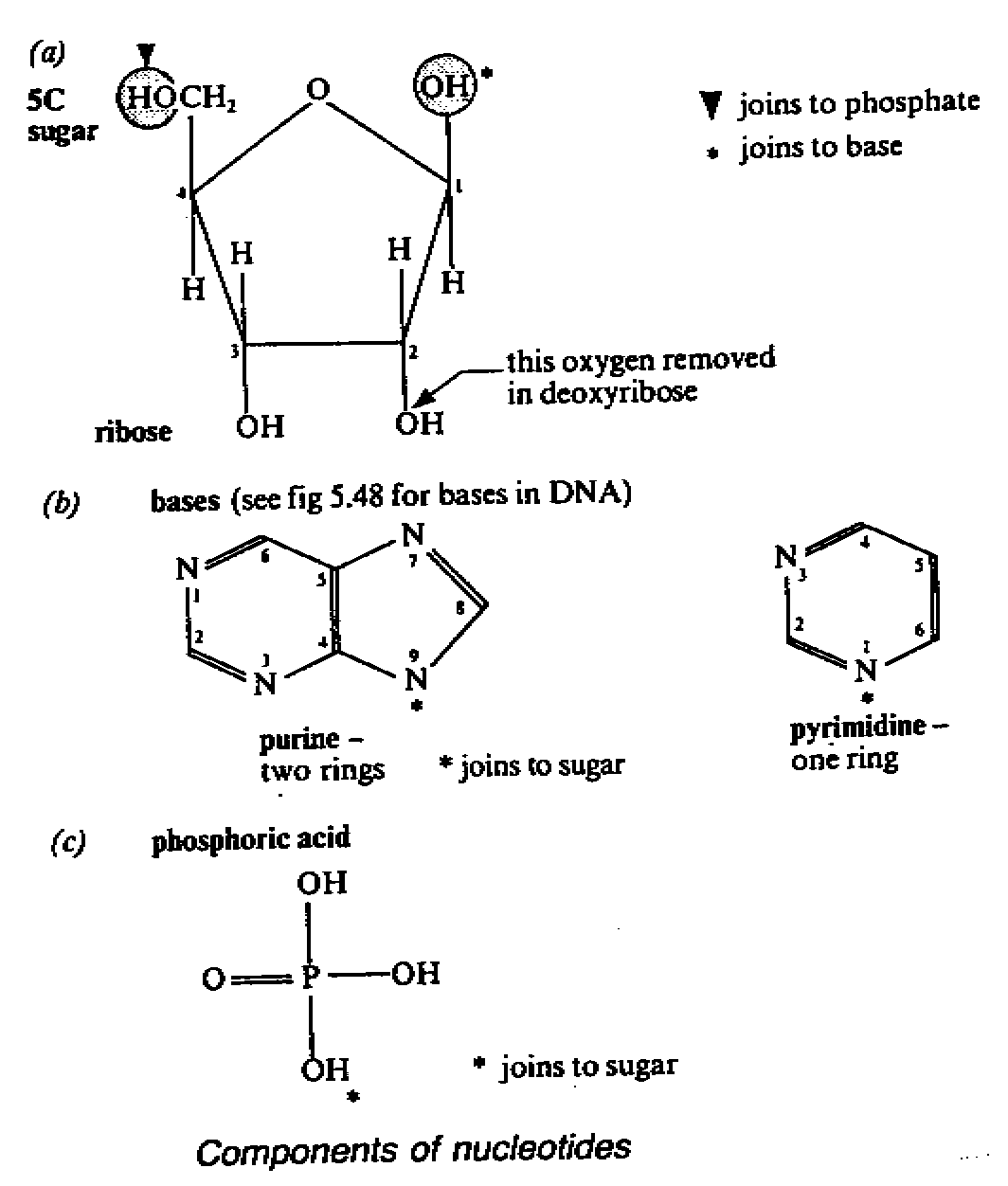
2. Base
- for each type of nucleic acids, there are 4 different bases. The nitrogen atom in the
rings give the
molecules their basic nature
- the bases are adenine(A), guanine(G), thymine(T) in DNA or uracil(U) in RNA and
cytosine(C)
- U,C and T belongs to a group of bases called
pyrimidine(single ring with 6 sides). A, G belongs to
a group of bases called purine(double ring
with a six-sided and a five sided rings)
3. Phosphoric acid they give nucleic acid their
acid character. The following figure shows how the
sugar, base and phosphoric
acid combine to form a nucleotide:
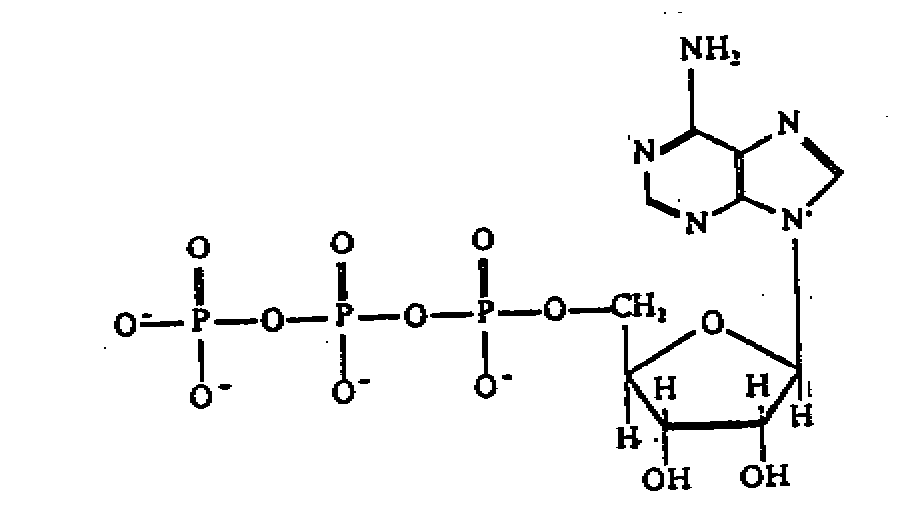
Mononucleotide
- a single nucleotide is called a mononucleotide e.g. ATP
- ATP is composed of the base purine adenine linked to
the 5 carbon sugar ribose with 3 phosphate
group
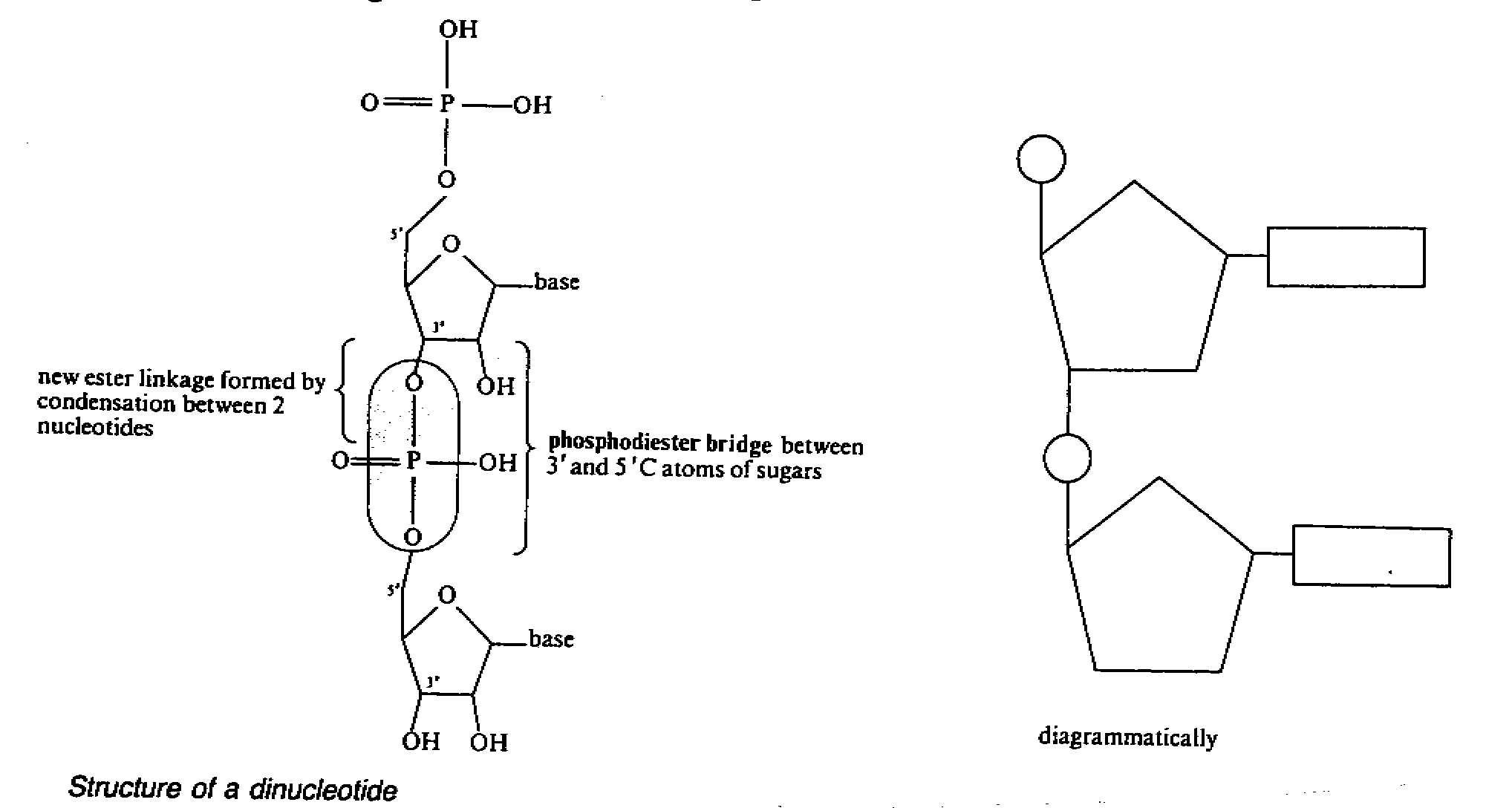
Dinucloetide
- two nucleotide join to form a dinucleotide by condensation
between the phosphate group of one with
the sugar of the other one. e.g. nicotinamide adenine
dinucleotide(NAD)
Polynucleotide
- by repeating the condensation process between phosphate group of
one nucleotide and the sugar of
the other for about several million times, a polynucleotide is
formed
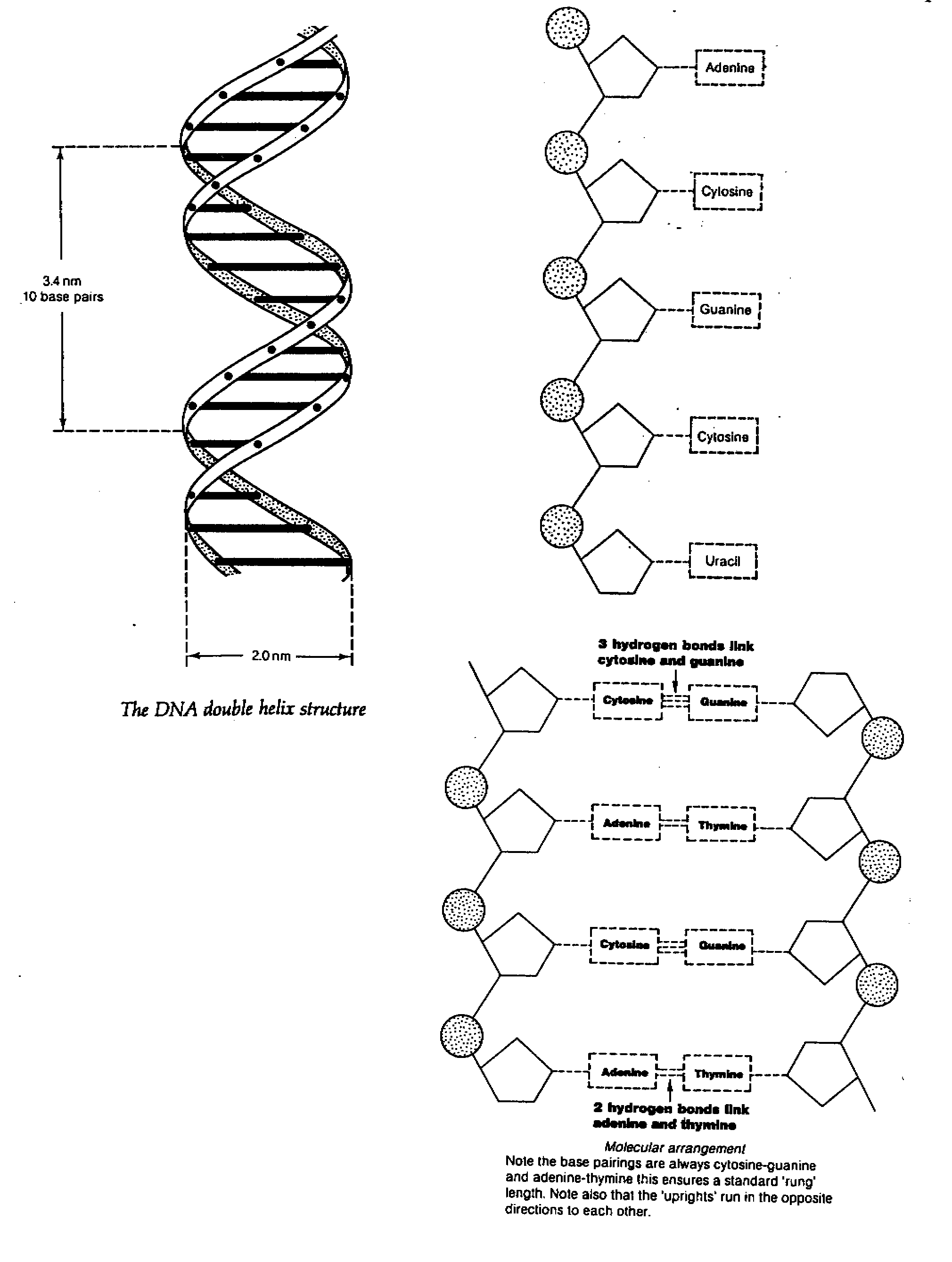
DNA
- it is a double-stranded polymer of nucleotide where the pentose sugar is always
deoxyribose and
organic bases are adenine, guanine, cytosine
and thymine but never uracil
- James Watson and Francis Crick suggested DNA has a structure of double helix of 2
nucleotide
strands, each strand being linked to the
other by pairs of organic bases joined by hydrogen bonds.
The pairing of organic bases are always C
and G, A and T
- The two chains that form the upright run in opposite directions i.e. antiparallel
RNA
- it is a single strand polymer of
nucleotide where the pentose sugar is always ribose and the
organic bases are A, G, C and U
but never T its structure is shown below:
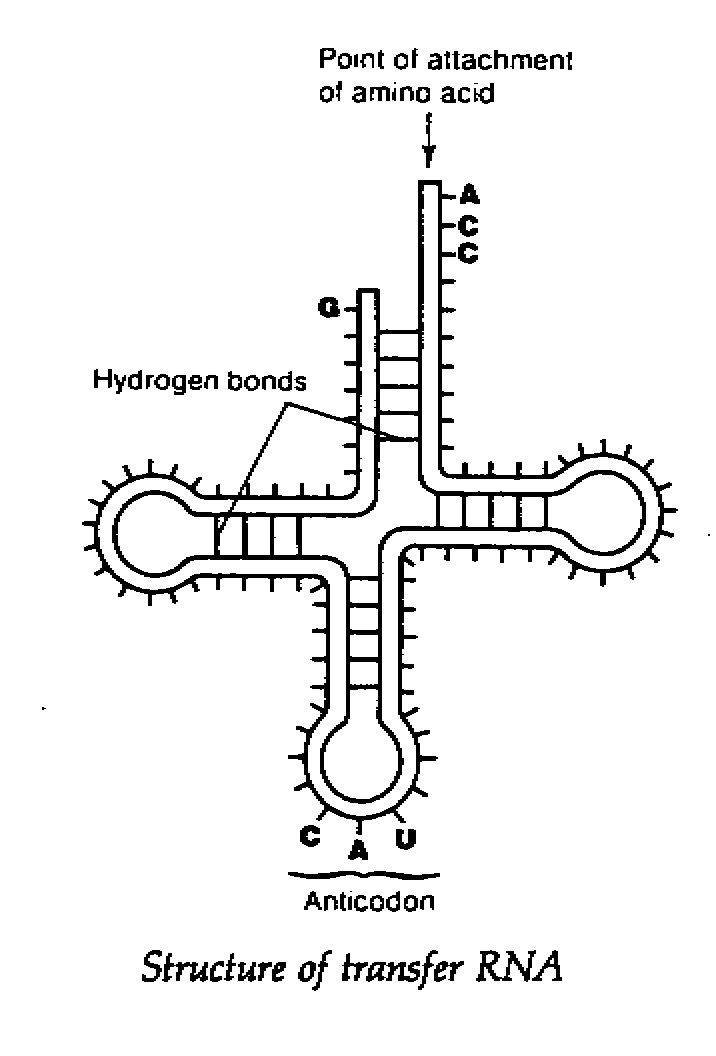
There are three types of RNA found in cells, all of which are involved in protein
synthesis
Ribosomal RNA(rRNA)
- it makes up 80% of total RNA of the cell, it combine with ribosomal protein to
form ribosome
- it is made by DNA of nucleus, but it is found in cytoplasm where it make up more than
half the
mass of ribosome
Transfer RNA(tRNA)
- it is a small molecule(about 80 nucleotide)comprising a single strand
- it is made by nuclear DNA and is responsible for carrying amino acids to mRNA during
protein
synthesis
Messenger RNA(mRNA)
- it is a long single-strand molecules (made of thousands nucleotide) which is formed
into a helix
- it is manufactured in the nucleus and is a mirror copy of part of one strand of the
DNA helix. It
enters cytoplasm where it associate with the
ribosomes and acts as a template for protein synthesis
Biological importance of water
l. Solvent properties
- it is an excellent solvent for polar substances, including ionic substances and some
non-ionic substances like sugar and alcohol
which contain charged (polar )groups such
as -OH group
- once molecule or ions are in solution they can move freely and so is more chemically
reactive. Thus majority of cells' chemical
reaction takes place in aqueous form
- non-polar parts of molecules are repelled by water and usually group together in its
presence i.e. non-polar molecules are hydrophobic(water-hating). This
hydrophobic interactions helps in maintaining the stability of membrane, many
protein molecules and nucleic acids
2. high heat capacity
- water has a high specific heat capacity (the amount
of energy required to raise the
temperature of 1 kg of water by 1 )This means that a large amount of energy
results in a relatively small rise in temperature. This causes temperature changes in
water to be minimised and so chemical reactions can take place over a small
temperature range. Water also provides a very constant external environment for
cells and organisms especially aquatic organisms.
3. High heat of vapourisation
- water has a latent heat of vaporisation and thus the energy imparted to water
molecules
to vaporise them results in loss of energy from their surroundings that is a cooling
effect occurs. This is made use of in sweating and panting in mammals and in
cooling transpiring leaves
4. High heat of fusion
- water requires relatively large amounts of
heat energy to thaw it. Coversely, liquid
water must lose a relatively large amount of heat energy to freeze. Contents of
cells and their environments are therefore less likely to freeze
5. Density and freezing properties
- the density of water decreases below 4 and ice
therefore tend to float. Since ice floats, it
can insulate the water below it, thus increasing the chance of survival of
organisms in the water. This is important for life in the cold climates and cold
seasons. Also water tends to rise below 40C helps to maintain circulation in
large
bodies of water. This may result in nutrient cycling of water to greater depth
6. High surface tension and cohesion
- this is important in the translocation of water
through the xylem
7. Water as a reagent
- it is an essential metabolise, particularly as a source of
hydrogen in photosynthesis and
is used in hydrolysis
reactions




