Please note that this material is copyright to the publishers of Coral Reefs and to the author
Not to be reproduced in any way without permission except for personal study

Coral Reefs (1983) 2: 101-110
Halimeda Biomass, Growth Rates and Sediment Generation on Reefs in the Central Great Barrier Reef Province*
Edward A. Drew
Australian Institute of Marine Science, P. M. B. No. 3, Townsville M.S.O., Oueensland 4810, Australia
* Contribution No.180 from the Australian Institute of Marine Science
Received 29 November 1982, accepted 1 May 1983
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Abstract
The average biomass of Halimeda per m2 of solid substratum increased progressively on a series of reefs situated at increasing distances from the shore in the central Great Barrier Reef. There was none on a reef close inshore, increasing to nearly 500 g m-2 total biomass ((90% caIcium carbonate) on an oceanic atoll system in the Coral Sea. The biomass measured contained 13 species of Halimeda but was dominated by only two species, H. copiosa and H. opuntia, except on the atoll where H. minima was dominant. Three sand-dwelling species were also present but did not occur anywhere in substantial quantities. Growth rates of the dominant species were measured by tagging individual branch tips. A mean value of 0.16 segments d-1 was recorded but 41 % of the branch tips did not grow any new segments whilst only 1% grew more than one per day. The number of branch tips per unit biomass was very constant and has been used in conjunction with growth rates and biomass to calculate productivity rates, and thence sedimentation, in the lagoon of one of the reefs. Biomass doubling time of 15 d and production of 6.9 g dry wt m-2 d-1 are considerably higher than previously reported values for Halimeda vegetation and there was little seasonal change detected over a whole year. Those values indicate annual accretion of 184.9 g m-2 year-1 of Halimeda segment debris over the entire lagoon floor (5.9 km2) of Davies Reef, equivalent to 0.13 mm year-1 due to Halimeda alone, or 1 m every 1,892 years when other contributions to that sediment are taken into account.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Introduction
Halimeda is a genus of calcified coenocytic green algae, almost exclusively tropical in distribution and currently placed In the Caulerpales (Chlorophyta) according to Hillis-Colinvaux (1980). Thirty species are distinguished according to the shape, size and internal structure of their segments which are joined together by small uncalcified nodes into variously branching chains to produce more or less bushy plants. In this study the segmented structure has been used as a basis for growth analysis.
Halimeda vegetation is often particularly well developed on coral reefs where different species may form either sand-dwelling communities anchored by substantial rhizoidal holdfasts, or lithophytic communities on dead coral, crustose coralline red algae, etc. They may be found between 0 and at least 70 m depth.and appear to be highly productive (Hillis-Colinvaux 1974). They also contribute considerable amounts of aragonitic calcium carbonate to reefal sediments, initially as discrete segments. According to Milliman (1974) and Stoddart (1969), unconsolidated carbonate sediments such as these are at present, and also have been in the geological past, quantitatively more irnportant to building reefs than is the carbonate incorporated into the framework organisms such as corals and crustose coralline red algae. The in situ production of calcium carbonate sands by Halimeda has been described by Wiman and McKendree (1975) and Neumann and Land (1975), whilst massive export of Halimeda debris from shallow water down to below 100 m depth on deep windward reef slopes has been documented by studies from submersibles (Ginsburg, and James 1973; Lang 1974; Moore et al. 1976). Extensive areas of predominantly Halimeda debris have also been documented for parts of the Great Barrier Reef Province (Maxwell 1969, 1973; Orme et al. 1979).
Quantitative studies of Halimeda standing crop, growth and productivity are scarce. In situ measurements of sediment accretion and plant growth have been reported for sand-dwelling species (Bach 1979; Merten 1971; Wefer 1980) and have shown, for instance, that up to one new segment per branch per day may be added to such plants. Laboratory studies of photosynthetic oxygen evolution (Hillis-Colinvaux 1974) and of the degree, mechanism and rates of calcification by Halimeda have been reported (Bohm 1973; Borowitzka and Larkum 1976 a,b,c, 1977; Stark et al. 1969). However, few attempts have been made to quantify the biomass of vegetation other than the relatively simple sand-dwelling communities. Larkum et al. (1967) included data for H. tuna in their study of lithophytic communities in Malta (Mediterranean Sea) but elsewhere the patchiness of this type of vegetation seems to have discouraged quantitation and indeed, Gilimartin (1960) reported abandoning such attempts at Enewetak Afoll for precisely that reason.
In this study Halimeda vegetation has been investigated on geographical scales ranging from individual plants to reefs many kilometres apart and on temporal scales ranging from days to years. The study area selected represents a potential gradient of physical, chemical and biological factors from the coast to the Coral Sea in the central region of the Great Barrier Reef Province. This gradient is at present subject of a multidisciplinary study, and the genus Halimeda is by far the most consistently conspicuous non-encrusting macroalga on the reefs involved.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
METHODS Biomass distribution-
Growth rates-
Data analysis
Methods
Biomass distribution
The study was made at a series of reefs situated at increasing distances from shore near Townsville ( 19o15' S, 146o18' E) in the central region of the Great Barrier Reef Province. The individual reefs were Pandora, Rib, Broadhurst, Davies, Myrmidon and Flinders Their locations are shown In Fig. 1. The last named is actually an oceanic atoll system detached from the eastern Australian continental shelf. Collections were made from three main habitats distinguished within individual reefs. These were the windward reef slope, lagoon bommies (otherwise called patch reefs, knolls or pinnacles) and the leeward reef slope (itself usually consisting of separate or coalesced bommies) These habitats are represented by positions 1, 3 and 4 respectively on the inset showing Davies Reef in Fig 1. Only three of the six reefs studied had lagoons. Little Halimeda was observed on reef flats which were therefore not sampled quantitatively.
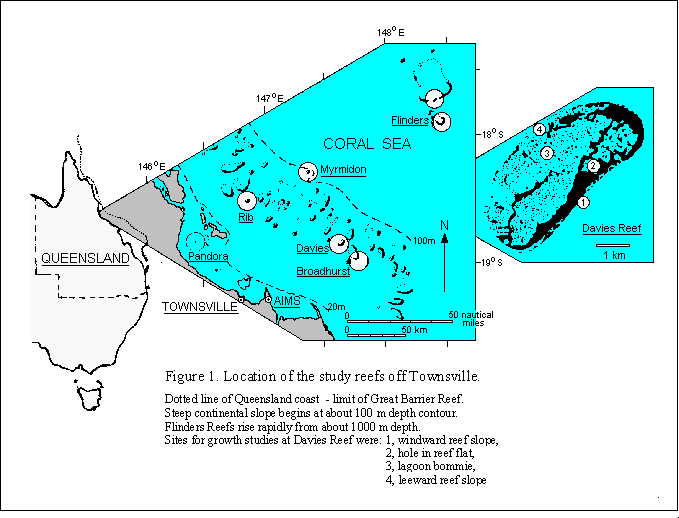
Biomass samples were collected only from solid substratum, derived either from coral or crustose coralline red algae and ranging in orientation from nearly horizontal to vertical or overhanging. SCUBA divers proceeded to the chosen depth (between 1 and 21 m below low water) where they collected any Halimeda present in a horizontal belt of contiguous 25 cm x 25 cm quadrats usually 6.25 m long. All the plants from one such sample were placed in a 0.5 cm mesh bag. Where the rock surface was considerably dissected, any Halimda growing in cavities directly beneath the quadrats, themselves oriented parallel to the substratum, was included. The number of quadrats sampled and the number without any trace Halimeda were recorded on a writing board on the collector's arm.
The representative nature of these samples was ensured by
(a) collecting at widely spaced sampling stations within the several km covered by different habitats on a reef.
(b) anchoring the diving boat at points selected only on the basis of availability of solid substratum.
(c) the very limited range of vision of the divers and the cryptic nature of much of the Halimeda vegetation which combined to minimise any initial bias introduced by the tendency to select a position with some Halimeda for the first quadrat of a 6.25 m long belt transect
Samples were air-dried on board ship and then, in the laboratory, they were each sorted into individual species. The resulting subsamples were dried to constant weight at 70 oC in a forced draught oven and weighed to +/-10 mg. Unidentifiable fragments, which seldom represented more than 10% of total biomass, were collectively weighed and that weight redistributed in proportion between all the identified species.
METHODS Biomass distribution-
Growth rates-
Data analysis
Growth rates
Halimeda grows by development of discrete new segments, usually at branch tips, and these rapidly achieve full size before calcification begins. The rate of addition of new segments should therefore be a good measure of growth. This was monitored at four localities on Davies Reef (see inset, Fig. 1 ) which supported substantial growths of the dominant lithophytic species H. copiosa and H. opuntia. Growth studies were restricted
to those two species
The tips of branches were tagged by folding a 3.5 cm length of wire-reinforced plastic twist-tie (white or red for easy relocation) between the second and third segments of those branch tips which had no lateral segments. This delimited a two segment portion as shown in Fig 3. Up to 12 batches of 10 tips were tagged at approximately two month intervals at each site. Each batch was restricted to an area no greater than 25 x 25 cm and included most of the suitable branch tips therein.
The sites, which were all situated between 5 and 8 m depth, were visited at approximately monthly intervals. At the first visit after tagging, groups of marked tips were relocated by means of flagging-tape markers tied close by, and the disposition of any new growth was drawn on underwater paper. At the second visit it was often difficult to find the tags without seriously disturbing the Halimeda vegetation, due to its prolific growth. In addition, some of the tips had by then developed new growth too complex to be drawn in situ. Therefore, at that visit all the tagged tips which could be located were collected for analysis ashore, and new batches were tagged nearby in undisturbed vegetation. Six separate but consecutive tagging periods of about 2 months duration were thus recorded during the year.
In the laboratory, new growth was recorded by detailed drawing. It was then separated from the plant fragment below it, dried and then weighed ( +/- 0.1 mg) and its total surrface area determined with a Licor LI-3000 area meter, with attached conveyor, to +/- 0.1 cm2. In order to keep track of the numerous individual plant fragments, each batch was initially taped to an A4 sheet of paper and then photocopied. This produced an excellent permanent record before subsequent dismembering for area and weight measurements.
METHODS Biomass distribution-
Growth rates-
Data analysis
Data Analysis and Presentation
Data for biomass and growth have been stored and analysed using a DEC PDP 11/70 computer. Statistical analyses were carried out using the software package MINITAB. Values are presented as mean +/- standard error of mean.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
RESULTS Species composition-
Biomass distribution-
Growth-
Productivity-
Sedimentation
Results
Species Composition
Sixteen species of Halimeda were found in collections from between 1 and 25 m below low water on the reefs studied. They were identified according to the key and descriptions set out by Hillis-Colinvaux (1980). Thirteen of the species occurred in varying amounts in the biomass samples collected from solid substrata, and these are listed in Table 1.
Table 1. Species composition of Halimeda biomass sampled at reefs increasingly distant from shore.(% dry weight for all samples at each reef, t indicates <0.05%,
distance measured from nearest shoreline on mainland or substantial high islands)
|
| Species |
All locations |
Rib |
Broadhurst |
Davies |
Myrmidon |
Flinders |
|
H. opuntia
(L.) Lamaroux |
39.7 |
43.9 |
43.8 |
42.6 |
25.2 |
11.6 |
H. copiosa
Goreau & Graham |
29.9 |
31.2 |
36.3 |
33.3 |
66.4 |
0.5 |
H. minima
(Taylor) Colinvaux |
10.7 |
0.0 |
0.0 |
0.0 |
1.2 |
55.3 |
H. micronesica
Yamada |
7.8 |
2.3 |
8.1 |
18.5 |
0.5 |
2.8 |
H. distorta
(Yamada) Colinvaux |
3.8 |
0.0 |
0.0 |
0.0 |
0.0 |
20.8 |
H. melanesica
Valet |
2.7 |
9.7 |
6.6 |
1.6 |
0.0 |
0.0 |
H. lacunalis
Taylor |
1.5 |
0.0 |
0.0 |
0.9 |
4.1 |
4.1 |
H. macrophysa
Askensay |
1.5 |
5.8 |
2.3 |
1.6 |
1.6 |
0.0 |
H. fragilis
Taylor |
0.8 |
0.0 |
0.0 |
t |
0.0 |
4.3 |
H. tuna
(Ellis & Solander) Lamaroux |
0.7 |
7.0 |
1.2 |
0.7 |
0.0 |
0.0 |
H. discoidea
Decaisne |
0.5 |
0.1 |
1.5 |
0.2 |
0.0 |
0.0 |
H. gigas
Taylor |
0.3 |
0.0 |
0.2 |
0.6 |
1.0 |
t |
H. taenicola
Taylor |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
0.6 |
|
|
All locations |
Rib |
Broadhurst |
Davies |
Myrmidon |
Flinders |
|
Total area (m2)
. |
236.9 |
20.6 |
29.8 |
137.4 |
28.9 |
20.2 |
Number of samples
. |
169 |
13 |
64 |
59 |
19 |
14 |
Total biomass (kg)
. |
36.0 |
1.1 |
4.4 |
13.4 |
7.7 |
9.4 |
Biomass (g m-2)
(standard error) |
228.4
(28.5) |
91.0
(20.5) |
190.8
(36.9) |
167.1
(21.5) |
250.5
(36.5) |
498.7
(82.8) |
Distance offshore (km)
. |
|
31 |
46 |
51 |
79 |
195 |
| % H. opuntia + H. copiosa |
69.6 |
75.1 |
80.1 |
75.9 |
91.6 |
12.1 |
|
|
Quantitative data were not obtained for the species from unstable substrata. Such species were restricted to small areas at the bases of bommies in lagoons and on leeward reef- slopes and to isolated sand pockets on windward reef slopes. H. cylindracea Decaisne and H. macroloba Decaisne formed the majority of this soft substratum group whilst H. stuposa Taylor was found once on the windward reef slope of Flinders Reef. In addition, H. discoidea grew on substantial pieces of coral debris in sand as well as on solid substrata.
Nearly 80% of the Halimeda biomass collected from solid substratum consisted of only two closely related species on all reefs except Pandora Reef, the innermost with no Halimedaa at all, and Flinders Reef, the outermost. The two species, H. copiosa and H. opuntia, were usually present in approximately equal proportions. On Flinders Reefs two other species, H. distorta and H. minima, made up a similarly major proportion of the total Halimeda biomass These latter two species are also both placed in the Opuntia group of the genus and they were either completely (H. distorta) or virtually (H. minima) absent from all the other reefs studied. With the occasional exception of H micronesica and H. melanesica, the remaining species were always very minor components of the Halimeda vegetation.
Note
Most of the material included as H. copiosa differed markedly from the Caribbean type material of that species as described by Goreau and Graham (1967) It had a sprawling habit, no discrete holdfast, and rather angular segments, but it was referred to H. copiosa by Hillis-Colinvaux (personal communication). A small amount of material showing all the characteristics of the type material of H. copiosa was also found and in this study the two have been combined. The latter species represented less than 5% of total H. copiosa at all sites except Flinders Reefs. where it was the only form found
RESULTS Species composition-
Biomass distribution-
Growth-
Productivity-
Sedimentation
Biomass distribution
Halimeda biomass and its species composition on the various reefs, is shown in Table 1. The values refer to total dry weight of the plants, of which 90% or more would be calcium carbonate (Bohm 1973). Mature segments of H. copiosa, which made up nearly half the blomass collected in this study, were, in fact, found to contain 91.3% ash.
Although the Halimeda vegetation was very patchy, at least a trace of it occurred in 46.6% of the 3,415 quadrats sampled altogether on Rib, Davies, Myrmidon and Flinders Reef. Frequency data are shown in Fig. 2 for those reefs but were not obtained for Broadhurst Reef. The minimum area which must be sampled in order to obtain an estimate of biomass within 33% of the overall mean for a given location was determined by selecting the biomass values for individual samples in random order and using them to calculate a cumulative mean biomass. Ten such analyses for each location indicated a mean minimum area of 8.3 +/- 2.3 m2 for all the locations sampled. This represents only six samples, each consisting of 25 quadrats.
There was a marked increase in the frequency of occurrence of Halimeda in quadrats on reefs further from the coast and this was accompanied by a considerable increase in mean biomass, as shown in Fig. 2.

Despite the considerable variation observed between samples at any one reef, 1-way analysis of variance showed the difference between the reefs to be significant at the 5% level of confidence.
The distribution of Halimeda biomass, and its species composition, between the three major intra-reef habitats investigated are set out in Table 2 A and B. The latter contains only data for Flinders Reefs because of their markedly different species composition. The variation in biomass between the habitats was not significant at the 5% confidence level (1-way analysis of variance) even when the Flinders Reefs data was included with that from the other reefs, although for Flinders Reefs the marked difference between windward reef slope and lagoon bommies was significant at the 2% confidence level (t-test).
Table 2A. Species composition of biomass from three habitats on reefs in the central Great Barrier Reef Province
(% dry weight; t indicates <0.05%; species in parentheses not present;
values = combined data from Rib, Broadhurst, Davies and Myrmidon Reefs)
|
| Species |
All locations |
Windward slope |
Lagoon bommiesa |
Leeward slope |
|
| H. opuntia |
40.2 |
38.9 |
42.8 |
27.7 |
| H. copiosa |
40.3 |
42.9 |
36.9 |
52.7 |
| H. minima |
0.2 |
0.7 |
0.0 |
0.0 |
| H. micronesica |
10.2 |
9.7 |
10.3 |
11.9 |
| (distorta) |
|
|
|
|
| H. melanesica |
3.8 |
1.3 |
5.3 |
3.1 |
| H. lacunalis |
1.0 |
2.1 |
0.5 |
0.5 |
| H. macrophysa |
2.1 |
2.5 |
1.9 |
1.8 |
| H. fragilis |
t |
t |
0.0 |
0.0 |
| H. tuna |
1.0 |
1.2 |
0.8 |
2.1 |
| H. discoidea |
0.7 |
0.1 |
1.1 |
0.1 |
| H. gigas |
0.5 |
0.6 |
0.4 |
0.1 |
| (taenicola) |
|
|
|
|
|
| Total area (m2) |
216.6 |
63.4 |
95.0 |
58.1 |
| Number samples |
155 |
52 |
81 |
22 |
| Total biomass (kg) |
26.7 |
10.9 |
12.4 |
3.3 |
Biomass (g m-2)
(standard error) |
180.7
(18.1) |
172.2
(31.7) |
205.1
(27.3) |
111.1
(16.8) |
% H opuntia + H copiosa |
80.5 |
81.8 |
79.7 |
80.4 |
|
| a includes samples totalling 10.9 m2 from deep holes on Davies Reef flat |
|
Table 2B. Species composition of biomass from two habitats at Flinders Reef
(% dry weight; t indicates <0.05%; species in parentheses not present)
|
| Species |
All locations |
Windward slope |
Lagoon bommiesa |
|
| H. opuntia |
11.6 |
1.1 |
17.3 |
| H. copiosa |
0.5 |
1.3 |
0.1 |
| H. minima |
55.3 |
58.2 |
53.5 |
| H. micronesica |
2.8 |
2.1 |
3.1 |
| H. distorta |
20.8 |
27.6 |
17.1 |
| (melanesica) |
|
|
|
| H. lacunalis |
4.1 |
4.6 |
3.9 |
| (macrophysa) |
|
|
|
| H. fragilis |
4.3 |
4.1 |
4.4 |
| (discoidea) |
|
|
|
| (tuna) |
|
|
|
| H. gigas |
t |
0.1 |
0.0 |
| H. taenicola |
0.6 |
0.9 |
0.5 |
|
| Total area (m2) |
20.2 |
12.3 |
8.0 |
| Number samples |
14 |
18 |
6 |
| Total biomass (kg) |
9.4 |
3.8 |
5.6 |
Biomass (g m-2)
(standard error) |
498.7
(82.8) |
311.1
(54.9) |
748.9
(118.7) |
% H minima + H distorta |
76.1 |
85.8 |
70.6 |
|
The small number of samples available for any particular depth and reef at present precludes quantitative analysis in that dimension. No major variation in species composition was apparent between habitats or with depth.
RESULTS Species composition-
Biomass distribution-
Growth-
Productivity-
Sedimentation
Growth
A total of 1685 branch tips of H. copiosa and H. opuntia were studied at four localities on Davies Reef. The data for the two species, which grew intermixed, developed in a similar manner and were often difficult to distinguish underwater, have been combined.
Only 57.5% of the tagged tips were recovered at the end of their respective tagging periods, which lasted between 40 and 73 d and spanned a whole year. Of those recovered, 40.6% had not grown at all. The 572 tips which had grown and were analysed further had put on a total of 8,623 segments. This represented a total area of 6,457 cm2 and dry weight of 265.3 g. An average segment in this new growth thus weighed 30.9 mg, had an area of 0.75 cm2 and a density of 41.1 mg cm2.
During four of the six consecutive tagging periods, new growth was recorded mid-way through as well as at the end of the period. Progressive development of a moderately fast-growing tip is shown in Fig. 3. The rate of segment accretion in all cases was very similar in both the intial and the total periods (Table 3) despite the greatly increased number of growth sites present on some branches at the beginning of the second part of the period.
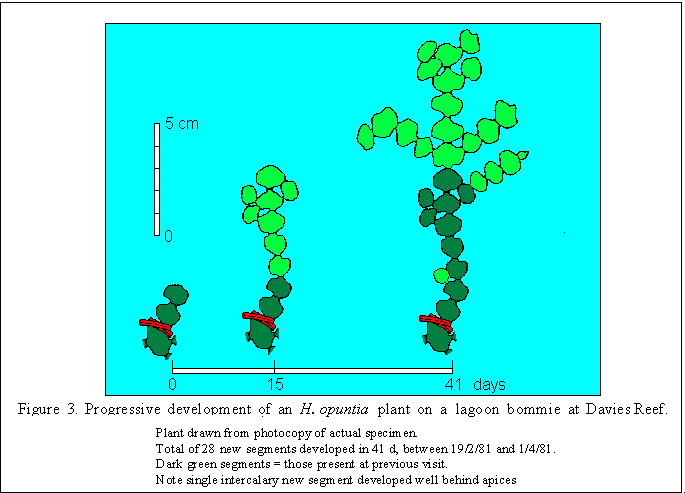
Table 3. Rates of segment production by H. copiosa and H.opuntia in first half and total tagging period in various habitats at Davies Reef
(Rate = new segments per surviving tagged tip per day)
|
| Period |
Site |
Initial |
|
Total |
|
Ratio |
|
|
Days |
Rate |
Days |
Rate |
|
|
| February/March |
Hole |
15 |
0.20 |
40 |
0.17 |
0.81 |
|
Bommie |
15 |
0.16 |
41 |
0.26 |
1.57 |
|
Leeward slope |
14 |
0.09 |
39 |
0.18 |
1.86 |
| April/May |
Hole |
20 |
0.18 |
62 |
0.12 |
0.64 |
|
Bommie |
22 |
0.22 |
63 |
0.11 |
0.48 |
|
Leeward slope |
22 |
0.16 |
62 |
0.14 |
0.89 |
| June/August |
Bommie |
39 |
0.16 |
72 |
0.17 |
1.06 |
|
Leeward slope |
39 |
0.11 |
73 |
0.11 |
1.05 |
| October/December |
Hole |
29 |
0.07 |
62 |
0.09 |
1.35 |
|
Bommie |
28 |
0.13 |
66 |
0.20 |
1.5 |
|
Leeward slope |
30 |
0.04 |
65 |
0.07 |
1.72 |
|
Mean
(standard error) |
|
|
0.14
(0.02) |
|
0.15
(0.02) |
1.18
(0.14) |
|
Only branch tips which had no lateral segments on the terminal two segments were tagged. In order to ascertain if these behaved differently from the entire range of branch tips present, their growth was compared with that of all those tips present on new growth at the start of the second period of growth. The latter could be individually monitored but were undisturbed and had not been selected subjectively. Those suitable for tagging in that group were 1.19 +/- 0.06 times more likely to grow at least one new segment than were the whole group. Those branch tips actually tagged were 1.53 +/- 0.16 times more likely to have grown at least one new segment in the first part of the period than were the whole group in the second part. This bias towards continued growth in the tips actually tagged has been allowed for in later considerations of segment production rates.
Of the tagged branch tips which grew new segments, a small number produced a large proportion of the new segments (Fig. 4) and the fastest growing tip produced 359 new segments in 68 days. Data from both the tagged material and from samples collected separately indicated a relatively constant distribution of branch tips within the vegetation, perhaps to be expected if the general morphology of the plants is to be maintained. There were 11.4 +/- 0.4 tips g-1 in 22 samples involving a total of 3,084 tips. Since an average segment in the tagged material weighed 30.8 +/- 0.9 mg, this indicates 1 tip or apical segment in every 2.8 segments. A similar value, 3.3 segments tip-1, was obtained from analysis of new growth on tagged branch tips (2,332 tips amongst 7,674 new segments).

An average production rate of 0.16 segments d-1 by all those tagged branch tips which survived and were relocated (Table 4 A) indicated a gap of about 8 days between inception of each new segment. However, if the considerable number of tips which did not grow at all are discounted, then new segments were developed at 4 to 5 d intervals and much more frequently on the very active branch tips. Data for one tagging period at Myrmidon Reef (Table 4 B) showed similar rates of segment production.
Table 4A. New segment development in H copiosa and H opuntia at Davies Reef
|
Period
(days) |
Location |
Total tips
tagged |
Recovered tips |
Growing tips |
|
|
|
total tips
(%) |
segments
d-1 |
Recovered
tips (%) |
segments
tip-1 d-1 |
|
| February/March |
Hole |
75 |
78.7 |
0.17 |
78.7 |
0.23 |
| (40) |
Bommie |
93 |
46.2 |
0.26 |
92.5 |
0.31 |
|
Leeward slope |
64 |
56.3 |
0.18 |
71.9 |
0.35 |
| April/May |
Seaward slope |
81 |
51.9 |
0.03 |
80.2 |
0.05 |
| (62) |
Hole |
95 |
60.0 |
0.12 |
73.7 |
0.21 |
|
Bommie |
80 |
45.0 |
0.11 |
85.0 |
0.16 |
|
Leeward slope |
73 |
35.6 |
0.14 |
91.8 |
0.18 |
| June/August |
Seaward slope |
19 |
26.3 |
0.07 |
89.5 |
0.12 |
| (73) |
Hole |
94 |
52.1 |
0.14 |
79.8 |
0.23 |
|
Bommie |
78 |
60.3 |
0.17 |
85.9 |
0.22 |
|
Leeward slope |
51 |
72.5 |
0.11 |
62.7 |
0.23 |
| August/October |
Hole |
121 |
66.9 |
0.05 |
62.8 |
0.12 |
| (55) |
Bommie |
200 |
79.5 |
0.31 |
70.0 |
0.50 |
|
Leeward slope |
120 |
57.5 |
0.08 |
61.7 |
0.23 |
| October/December |
Hole |
95 |
69.5 |
0.09 |
69.5 |
0.16 |
| (62) |
Bommie |
96 |
83.3 |
0.20 |
70.8 |
0.30 |
|
Leeward slope |
40 |
67.5 |
0.07 |
70.0 |
0.13 |
| December/February |
Hole |
110 |
30.0 |
0.09 |
89.1 |
0.15 |
| (73) |
Bommie |
100 |
49.0 |
0.12 |
75.0 |
0.24 |
|
| Means |
Seaward slope |
100 |
|
0.05+/-0.03 |
|
0.09+/-0.05 |
|
Hole |
590 |
|
0.11+/-0.02 |
|
0.18+/-0.01 |
|
Bommie |
647 |
|
0.22+/-0.03 |
|
0.29+/-0.04 |
|
Leeward slope |
348 |
|
0.11+/-0.01 |
|
0.23+/-0.04 |
|
|
Overall |
1,685 |
|
0.16+/-0.01 |
|
0.27+/-0.04 |
|
Table 4B. New segment development in H copiosa and H opuntia at Myrmidon Reef
|
Period
(days) |
Location |
Total tips
tagged |
Recovered tips |
Growing tips |
|
|
|
total tips
(%) |
segments
d-1 |
Recovered
tips (%) |
segments
tip-1 d-1 |
|
| November/January |
Seaward slope |
90 |
75.6 |
0.09 |
44.1 |
0.21 |
| (62) |
Leeward slope |
70 |
87.1 |
0.06 |
45.7 |
0.16 |
|
|
Overall |
160 |
|
0.07+/-0.02 |
|
0.19+/-0.03 |
|
There was no significant seasonal difference between the rates of new segment production in any of the three habitats investigated throughout the year (1 -way analysis of variance, 5% confidence level). However, segment production on the lagoon bommie was greatest during 5 of the 6 sampling periods and the mean annual rate of new segment production there was very significantly higher ( 1% confidence level) than in the other habitats.
Difficulties were experienced in sampling the reef front where there were very few suitable tips for tagging. perhaps due to grazing pressure. The data from only two effective tagging periods there may be un-representatively low and has not been analysed further.
The mechanisms by which tagged branch tips were lost during the experiments were not elucidated but grazing by large and small fish and a range of crustaceans and molluscs, plus post-reproductive disintegration (Hillis-Colinvaux 1980) could account for losses varying from parts of segments to whole plants and even patches of Halimeda vegetation. The survival of tagged branch tips throughout the tagging experiment is shown in Fig. 5. The rate of loss was surprisingly linear at about 0.6% d-1. The 171 branch tips which were lost after their growth had been recorded midway through the tagging period had been growing at 0.14 segments d-1. This indicates that lost tips were no different in growth characteristics from those which survived, so that the entire tagged population can be considered as one, whether recovered or not.

RESULTS Species composition-
Biomass distribution-
Growth-
Productivity-
Sedimentation
Productivity
The lagoon at Davies Reef is a well defined region in which the most extensive measurements of Halimeda biomass and growth were made. This allows Halimeda performance there to be considered in some detail. Two approaches to calculating rates of`production from the available data are set out in Table 5.
Table 5. Calculation of Halimeda productivity on the sides of bommies in Davies Reef lagoon (rate of sediment production adjusted for bias of 1.53 towards growth occurring in tips actually tagged)
|
| A. |
Doubling time |
|
1 g biomass contained 11.4 +/- 0.4 branch tips |
|
1 branch added 0.14 +/- 0.03 segments d-1 |
|
1 segment = 30.8 +/- 0.9 mg dry wt therefore 4.3 +/- 1.1 mg d-1 added per branch |
|
1 g biomass therefore produced 49.0 +/- 3.4 mg dry wt d-1 |
|
1 g new biomass produced exponentially in 15 d
|
|
Productivity |
|
176.6 +/- 24.2 g dry wt m-2 biomass on bommies contained 2013 +/- 356 branch tips
|
|
1 branch tip added 0.14 +/- 0.03 segments d-1 |
|
.................................. = 4.3 +/- 1.1 mg dry wt d-1 |
|
Total production = 4.3 +/- 1.1 x 2013 +/- 356 mg dry wt m-2 d-1 |
|
.................................. = 8.7 +/- 4.1 g dry wt m-2 d-1 |
|
|
| B. |
Rate of loss |
|
50% of tagged tips lost in 80 d (Fig. 5) |
|
basal 2 must be lost to lose tag |
|
average lost tip= 2 + (80 x 0.14 +/- 0.03) segments |
|
.................................. = 13.2 +/- 2.5 segments |
|
.................................. = 406.6 +/- 91.1 mg dry wt |
|
2013 +/- 356 such tips per m2 and linear rate of loss = 0.58% d-1 (Fig. 5) |
|
Total rate of loss = 4.8 +/- 2.1 g dry wt m-2 d-1
|
|
The first is based on the number of branch tips per unit biomass and the rate of production of new segments. This indicated a doubling time for total biomass of 15 d, an important ecological parameter which is independent of the actual biomass present. Biomass must be lost at a similar rate, otherwise production throughout the year at the rate observed would lead to an exponential increase to an immense standing crop. It is not unreasonable to assume that losses in this situation may equal growth to give a moderately constant biomass, at least from year to year. It follows that about 3.2 +/-1.5 kg dry weight of Halimeda was produced, and also lost annually per m2 of solid substratum on lagoon bommies.
Alternatively, the rate of loss of tagged branches was directly measured and can be used in conjunction with rates of new segment production to estimate the loss of material. This will necessarily be a minimal value as loss of only the tagged part of each branch is assumed. Loss of 1.8 +/- 0.8 kg m-2 year-1 was indicated by these calculations and again, to maintain the anticipated constant biomass, production must have equalled that.
Thus, on average, this Halimeda vegetation produced 6.8 +/- 3.1 g dry wt m-2 d-1 (2.5 +/- 1.1 kg m-2 year-1) of segments. Assuming only 10% of this to be organic matter
in the species involved (Bohm 1973, own measurernent for H. copiosa) and this organic matter to be 47% carbon (Westlake 1963), then daily production of 6.1 +/- 2.8 g m-2 CaCO3, 0.7 +/- 0.3 g m-2 organic matter or 320 +/- 146 mg m-2 carbon occurred. Since many of the segments present
in this Halimeda vegetation weighed up to twice as much as the relatively young ones involved in the growth experiments, these estimates are necessarily minimal.
RESULTS Species composition-
Biomass distribution-
Growth-
Productivity-
Sedimentation
Sedimentation
Computer-assisted planimetry of aerial photographs of Davies Reef indicated a perimeter of 54.6 km for the lagoon and the many bommies enclosed therein. Most of these structures had near-vertical sides extending from low water to sand/rubble bottom at 8 to 10 m depth. Therefore, an area of 4.9 x 106 m2 of solid substratum was available for Halimeda growth which would bear 8.7 x 104 kg of that vegetation, mostly as H. opuntia and H. copiosa. Productivity calculations set out above indicate that this would yield 10.9 x 105 kg of calcareous sediment per year. In such a lagoon with a bottom area of 5.9 x 106 m2 , an annual accretion of 184.9 g m-2 would occur. Sediments from Davies Reef lagoon have a density of 1.40 +/- 0.01 g cm-3 (gravimetric determination) so that 0.13 mm vertical accretion would occur per year over the
whole lagoon floor due to Halimeda alone.
Sedimentological data (S. Tudhope, personal communication) indicate that Halimeda debris contributed about 25% of Davies Reef lagoon sediments with somewhat more immediately adjacent to bommies. If it is tentatively assumed that the sediment composition reflects the rate of contribution of the various components, total accretion at the present time of 0.52 mm, or 1 m every 1,892 years, can therefore be expected. However, since this accretion progressively reduces the solid substratum available for growth of Halimeda and other sediment generating organisms, rates of sedimentation may have been higher in the past and may decrease in the future. Furthermore, accumulation of the lagoon floor sediments will move them into shallower depths which may lead to greater export of sediment produced within the lagoon, due to water turbulence. No quantitative data are available on the present rate of sediment export but with the lagoon floor mostly deeper than 15 m, this is probably low. Import of sediment to the lagoon from the seaward reef slope and reef flat has not been included in these considerations.
The Halimeda biomass within this lagoon, 176.6 g m-2 suitable substratum, was by no means the highest recorded in this study. However, the presence of a large number of bommies within a restricted area was a major factor allowing a high total biomass within the lagoon, resulting in the substantial sediment accretion indicated above. Nevertheless, considerable rates of Halimeda sediment accretion must be occurring throughout the area studied whilst the role of Halimeda segments as a reef-framework
filler may be equally important.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Discussion
Sixteen species of Halimeda were found in the study area. There were no unconsolidated seagrass/Halimeda flats, such as those frequently encountered on Caribbean reefs, so that results cannot be directly compared with, for instance, the Jamaican situation where Hillis-Colinvaux (1980) found 10 species in one area. Flinders Reefs, an oceanic coral atoll system, is probably comparable with Enewetak Atoll where Hillis-Colinvaux (1980) reported the highest Halimeda diversity (14 species) for any specific area in the world. Flinders Reefs had only 3 species fewer and the 11 species present were all also reported for Enewetak Atoll, whilst one of those missing from Flinders Reef (H. macrophysa) did occur on the adjacent continental shelf reefs. Thus, these two coral reef areas,on different sides of the equator and nearly 6,000 km apart, have a very similar Halimeda flora (Table 6).
Table 6. Halimeda species at Enewetak Atoll (N. Pacific), Flinders Reefs (Coral Sea) and in the central region of the Great Barrier Reef Province.
(Enewetak data from Hillis-Colinvaux, 1980; GBR= Great Barrier Reef; Flinders Reefs and GBR data include species observed there but not collected in biomass samples)
|
|
Enewetak Atoll |
Flinders Reefs |
Central GBR |
|
| H. copiosa |
+ |
+ |
+ |
| H. cylindracea |
+ |
+ |
+ |
| H. discoidea |
O |
O |
+ |
| H. distorta |
+ |
+ |
O |
| H. fragilis |
+ |
+ |
+ |
| H. gigas |
+ |
+ |
+ |
| H. gracilis |
+ |
O |
O |
| H. incrassata |
+ |
O |
O |
| H. lacunalis |
+ |
+ |
+ |
| H. macroloba |
O |
O |
+ |
| H. macrophysa |
+ |
O |
+ |
| H. melanesica |
O |
O |
+ |
| H. micronesica |
+ |
+ |
+ |
| H. minima |
+ |
+ |
+ |
| H. opuntia |
+ |
+ |
+ |
| H. stuposa |
+ |
+ |
O |
| H. taenicola |
+ |
+ |
+ |
| H. tuna |
O |
O |
+ |
|
| Total |
14 |
11 |
14 |
|
The biomass of Halimeda per unit area of solid reef sampled clearly increased from close inshore to the Coral Sea, with none on Pandora Reef in very turbid inshore waters up to 500 g m-2 or more in the oceanic atoll situation. A substantial biomass was already present at Myrmidon Reef but the further increase to Flinders Reefs in the open ocean was accompanied by a dramatic change in species composition, making direct comparison more difficult. Preliminary oceanographic studies have established that periodic upwelling of deeper, nutrient rich water occurs both at the shelf break in this region (Andrews and Gentien 1982) and also in the region Flinders, Reef(J. C. Andrews, personal communication). It is possible that the higher Halimeda biomass, reflects localised addition of inorganic nutrients. The abundance of reef-associated grazing fish may also affect, or reflect, algal abundance. Williams (1982) showed that, like Halimeda, populations of such fish increased from shore to Coral Sea, although they were somewhat reduced at Flinders Reefs, the very seaward end of the transect. However, few of these fish graze directly on Halimeda (Williams, personal communication but prefer the fine algal turf so that their abundance may reflect growth of other algae paralleling that of Halimeda. Herbivorous grazing may also affect the biomass of this alga and the populations of grazing fishes are certainly lower at Flinders Reefs than elsewhere along the gradient (Williams 1982) except very close inshore.
The growth studies spanned a whole year but were restricted to one reef. They did, however, involve the two quantitatively most important species along most of the gradient studied and these were sprawling lithophytic species whereas previous studies on Halimeda have involved discrete, sand-dwelling species. The two species studied at Davies Reef showed tremendous variability in growth of apparently similar, healthy branch tips. Many did not grow at all whilst a few added several segments per day and branched copiously. Wefer( 1980) obtained similar results with H. incrassata in Bermuda, which grew seasonally and only when water temperature exceeded 20oC. The absence of seasonality in Halimeda growth at Davies Reef was probably due to the higher water temperature there which seldom dropped below 23oC or exceeded 30oC(J.C. Andrews, personal communication). For much of the year water temperature was close to the optimum for the genus, 27 - 29oC (Hillis-Colinvaux 1980). Wefer (1980) estimated a doubling time of 28 d for his population when it was growing rapidly, and a contribution to the surrounding sediment of 50 g m-2 year-1 calcium carbonate. Such growth rates, considerably less than those at Davies Reef, might be expected in the colder, sub-tropical locality. Bach (1979), again using sand-dwelling species but in somewhat warmer waters around Florida, estimated 25% loss of new growth as dead segments in 56 d, similar to the rate in Davies Reef Lagoon. However, his values of 4.2 g m-2 year-1 calcium carbonate production (50% by Halimeda) and 8.6 g m-2 year-1 organic matter (67% by Halimeda)and 8.6 g m-2 year-1 organic matter (67% by Halimeda) were very much lower than those from the present study. This may be due in part to his low standing crop of 8.33 g m-2 Halimeda (76% of total calcareous green algae present), an order of magnitude lower than that of Davies Reef, but it must be remembered that the Halimeda vegetation localised on the lagoonal bommies there contributed sediment to the extensive lagoon floor, not just to the immediate substratum on which the algae were growing. Merten (1971) measured increase in plant length rather than segment number in her populations of H. macroloba in Guam, where the temperature regime was slightly higher than at Davies Recf (27.4 to 33oC). She did, however, estimate only 3 crops per year but presented data indicating an average contribution of 404.9 g m-2 year-1 calcium carbonate to the sediment around those plants. Each m2 of actual vegetation on the bommies in Davies Reef lagoon produced 2,234 g m-2 year-1 calcium carbonate, still more than five times her value.
Thus, the sprawling, lithophytic species which dominate theHalimeda vegetation in the central region of the Great Barrier Reef appear to be considerably more productive than do the sand-dwelling species elsewhere under comparable environmental regimes. Reports by Maxwell (1968, 1973) that extensive areas of Halimeda-rich sediments occur in both the southern and northern regions of the Great Barrier Reef Province, and by Orme et al. (1980)that in the north extensive banks of Halimeda segments cover many km2 of seabed between Lizard Island and the outer shelf reefs suggest rapid production ofHalimeda may also be occurring outside the confines of the individual reefs and at 25 to at least 60 m depth.
Acknowledgements
The assistance of Kay Abel, Caroline Waid, Bret Cullen and Drew Thompson, both as diving assistants and in the laboratory, is gratefully acknowledged, as is the patience and help of the Masters and crews of R. V. Sirius and R. V. The Harry Messel. B.G. Hatcher and S. Tudhope made helpful comments on the manuscript.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
References
- Andrews J C, Gentien P (1982)
- Upwelling as a source of nutrients for the Great Barrier Reef ecosystems: a solution to Darwin's question?
Mar Ecol Prog. Ser 8:257 269)
- Bach S D (1979)
- Standing crop, growth and production of calcareous
Siphonales (Chlorophyta) in a South Florida Lagoon.
Bull Mar Sci 29: 191-201
- Bohm E L (1973)
- Studies on the mineral content of calcareous alga
Bull Mar Sci 23: 177 - 190
- Borowitzka M A, Larkurn A W L (1976a)
- Calcification in the green alga Halimeda. II The exchange Ca2+ and the occurrence of age gradient in calcification and photosynthesis.
J Exp Bot 27: 864 - 878
- Borowitzka M A, Larkurn A W L (1976b)
- Calcification in the green algaHalimeda. III. The sources of inorganic carbon for photosynthesis and calcification and a model of the mechanism of calcification.
J Exp Bot 27: 879 - 893
- Borowitzka M A, Larkurn A W L (1976b)
- Calcification in the green algaHalimeda.
IV. The action of metabolic inhibitors on photosynthesis and calcification.
J Exp Bot 27: 894 - 907
- Borowitzka M A, Larkurn A W L (1977)
- Calcification in the green alga Halimeda.
I. A ultrastructural study of the thallus development.
J Phycol 13: 6 - 16
- Gilmartin M (1960)
- The ecological distribution of the deep water algae of Eniwetok Atoll.
Ecology 41: 210 - 221
- Ginsburg R N, James N P (1973)
- British Honduras by submarine.
Geotimes 18: 23 - 24
- Goreau T F, Graham E A (1967)
- A new species of Halimeda from Jamaica.
Bull Mar Sci 17: 432 - 441
- Hillis Colinvaux L (1974)
- Productivity of the coral reef alga Halimeda (Siphonales).
Proc. 2nd Int Coral Reef Symp 1: 35 - 42
- Hillis Colinvaux L ( 1980)
- Ecology and taxonomy of Halimeda: primary producer of coral reefs.
Adv Mar Biol 17:1 - 327
- Lang J (1974)
- Biological zonation at the base of a reef.
Am Sci 62: 272 - 281
- Larkum A W D, Drew EA, Crossell R N (1967)
- The vertical distribution of attached marine algae in Malta.
J Ecol 55: 361 - 371
- Maxwell W G H (1968)
- Atlas of the Great Barrier Reef.
Elsevier, Arnsterdam
- Maxwell W G H (1973)
- Sediments of the Great Barrier Reef Province.
In: Jones 0 A, Endean R E (ed) Biology and geology of coral reefs, vol 1: Geology, Academic Press, New York London, pp 299 - 34
- Merten M J (1971)
- Ecological observations of Halimeda macroloba Decaisne (Chlorophyta) on Guam.
Micronesica 7: 27 - 44
- Milliman J D (1974)
- Recent sedimentary carbonates, pt 1: Marine carbonates.
Springer, New York Heidelberg Berlin
- Moore C H, Graham E A, Land L S (1976)
- Sediment transport and dispersal across the deep fore-reef and island slope (-55 m to -305 m), Discovery Bay, Jamaica.
J Sediment Petrol 46:1 74 - 187
- Neumann A C, Land L S (1975)
- Lime mud deposition and calcareous algae in the Bight of Abaco, Bahamas: a budget.
J Sediment Petrol 46: 174 - 187
- Orme G R, Flood P G, Sargent G E G (1978)
- Sedimentation trends in the lee of outer (ribbon) reefs,
Northern Region of the Great Barrier Reef Province.
Phil Trans R Soc London Ser A 291: 85 - 99)
- Stoddart D R (1969)
- Ecology and morphology of recent coral reefs.
Biol Rev 44: 433 - 498
- Stark L M, Almodovar L, Kraus R W (1969)
- Factors affecting the rate of calcification in Halimeda opuntia (L.) Lamouroux and Halimeda discoidea Decaisne.
J Phycol 5; 305 - 312
- Wefer G (1980)
- Carbonate production by algae Halimeda , Penicillus and Padina.
Nature (London) 285: 323 - 324
- Westlake D F (1963)
- Comparisons of plant productivity.
Biol Rev 38: 385 - 425
- Williams D McB (1982)
- Patterns in the distribution of fish communities across the Great Barrier Reef.
Coral Reefs 1: 35 - 43
- Wiman S K, McKendree W G (1975)
- Distribution of Halimeda plants and sediments on and around a patch reef near 0ld Rhodes Key, Florida.
J Sediment Petrol 45: 415 - 421
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References





