Please note that this material is copyright to the publishers of Coral Reefs and to the authors
Not to be reproduced in any way without permission except for personal study

Coral Reefs (1988) 6: 207-218
Studies on Halimeda II.
Reproduction, particularly the seasonality of gametangia formation, in a number of species from the Great Barrier Reef Province *
Edward A. Drew and Kay M. Abel
Australian Institute of Marine Science, PMB No. 3, M.S.O., Townsville, Queensland 4810, Australia
*Contribution No. 368 from the Australian Institute of Marine Science
Accepted 3 September 1987
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Abstract.
Seventeen species of Halimeda have been grown in the laboratory in an open-circuit seawater cascade during a 6 year period. All but one grew vegetatively (generated new segments) to some extent and several species grew luxuriantly. A few species also reproduced vegetatively by developing new plants at the ends of fine runners. However, most significant was the production of gametangia by all the species in the cascade whilst several of those species, plus one not grown in the cascade, were also observed fertile in the field. Different material from a single collection sometimes became fertile in the cascade in successive years and then often at very similar times, suggesting a distinct seasonality and response to environmental stimuli. Some lunar periodicity was also detected in the fertility of three species. Species-specific differences in the structure of gametangial clusters, particularly the size of the gametangia, were observed. In all species examined, gametophore branches ended in unexpanded tips which often had a distinct, thickened cap. Upon maturity, the contents of all the gametangia in a cluster were explosively released through these discharge papillae. This release usually commenced only 5 to 15 minutes after the plants were re-illuminated on the day following the appearance of gametangia although two species, H. incrassata and H. melanesica, often released their gametes in the late afternoon.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Introduction
Halimeda is a genus of green algae characterised by a macroscopic, segmented and calcareous thallus. The plants are coenocytic and are currently placed in the order Bryopsidales of the Chlorophyta (Hillis-Colinvaux 1984). They are of considerable importance in coral reef areas, contributing both organic production and significant amounts of calcareous sediment (Milliman 1974, Drew 1983; Drew and Abel 1985). They are known to reproduce by both vegetative and sexual processes. The scant information available about these processes has been summarised by Hillis (1959) and Hillis-Colinvaux (1980) who also noted that sexual reproductive structures had been described for only 16 species, 60% of the total currently recognised.
Vegetative reproduction involves the development of achlorophyllous filamentous rhizoids from either the holdfast region or from points on the thallus segments where growth of the medullary siphons would otherwise have produced new photosynthetic segments. In species normally found on soft substratum the rhizoids may grow several centimetres through the water or substratum to produce new apparently discrete plants some distance from the parent. In the sprawling lithophytic species of the section Opuntia, development of rhizoids from segments results in the formation of multiple holdfasts which may be followed by thallus fragmentation.
Stalked clusters of spherical globules on the surface of H. tuna thalli were first reported by Derbes and Solier (1856) but it was not until Nasr (1947) observed fusion of the biflagellate zooids released therefrom that their identity as gametangia was established, and even now the lifecycle from zygote to a new Halimeda plant has not be completed in culture. Meinesz (1972) did grow a small filamentous thallus from H. tuna zygotes, but it developed no further during the 5 month study. Particularly striking in the known life-cycle of Halimeda, in common with some other siphonous green algae, is the release of the entire protoplasmic contents of the segments as gametes. This is called holocarpy and is rapidly followed by death in Halimeda and disintegration of the resulting white calcified thallus into individual segments as the cell wall material forming the uncalcified nodes decomposes. Hillis-Colinvaux (1980) has likened this to the breeding strategy of an annual weed and also to the "strawberry-coral" model of Williams (1975) although the validity of these direct analogies is debatable.
In this paper we report new observations on the growth and reproduction of 18 species of Halimeda both in an open-circuit seawater cascade at our laboratory in tropical Australia, near Townsville, and also in the field, on coral reefs and inter-reefal Halimeda meadows within the Great Barrier Reef (GBR). In particular we demonstrate the apparent seasonality of gametangia formation in some of these species, describe in detail the gametangial clusters including those of several species not previously reported fertile, and discuss the process of gamete release.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Methods
This study was carried out over a 6 year period (1981-1987). Most of the plants used were collected from reefs in the Central Section of the GBR at about 19o S: 147o E. They were collected by Scuba divers from depths of 5-15 m on the windward reef slopes and lagoonal patch reefs (bommies) at Davies Reef, Keeper Reef, John Brewer Reef and Myrmidon Reef and also from the fringing reef flat at Cockle Bay, Magnetic Island. In the last 2 years of the study, living material of certain species was also obtained from the Southern section of the GBR at Heron Island (23o27' S: 151o58' E) and several localities in the Swains Reefs (about 22o S: 153o E), from the Northern section of the GBR at about 30 m depth in inter-reefal Halimeda meadows near the Ribbon Reefs off Cooktown (15o30' S: 145o45' E), and from the Far North section of the GBR near Tijou Reef (13o11' S: 143o51' E). All these localities are shown in Fig. 1. The sections of the GBR named above are based on the official zoning of the Great Barrier Reef Marine Park but for simplicity we shall refer to them as the southern, central and northern sections, the last combining the Northern and Far North sections.
 |
Figure 1
Great Barrier Reef locality map
AIMS = site of cascade system;
1a = Davies Reef;
1b = Keeper Reef;
1c = John Brewer Reef;
1d = Myrmidon Reef;
1e = Cockle Bay (Magnetic Island);
2 = Swains Reefs (3 localities);
3 = Heron Island;
4 = Ribbon Reefs (Cooktown);
5 = Tijou Reef
|
Living plants collected in the field were transported to the laboratory in seawater at ambient sea temperature and shaded from direct sunlight. Individual plants were attached with plastic cable-ties to racks made from 2.5 cm plastic garden trellis mesh. This was done either in the field or in the laboratory, and the racks were then suspended vertically in an open-circuit unfiltered seawater cascade screened from direct sunlight by 80% horticultural shade-cloth and window glass. Various aspects of the cascade are illustrated in Fig. 2. The racks were labelled with the name of the species together with the date, locality and habitat of collection. During growth in the cascade, we preserved the identity of individual collections, that is, groups of plants collected on the same day at the same reef or specific habitat thereon (windward reef slope, reef flat or lagoon bommie). However, due to vigorous growth, it was often necessary to split individual plants into several fragments during the period they grew in the cascade, and their exact relationship to other fragments within the collection was not recorded. All discrete fragments will hereafter be referred to as plants.
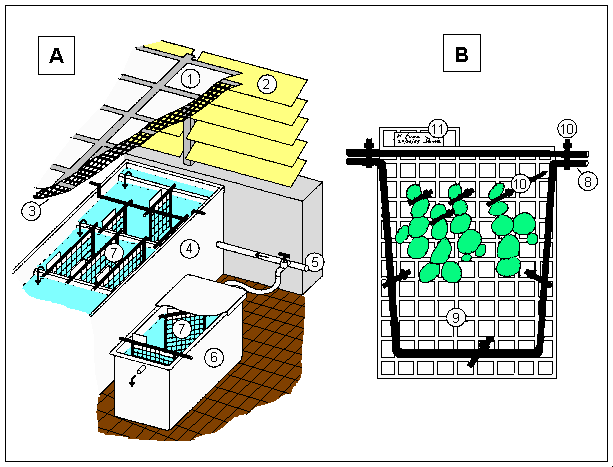
|
Fig. 2. The open circuit seawater cascade and plant transport system (A), and growth rack (B)
A
1 = wire-reinforced window glass, 2 = aluminium louvres, 3 = 80% horticultural shade cloth, 4 = glass-fibre sheathed cascade tanks, 5 = unfiltered seawater supply, 6 = portable semi-opaque polythene Nally bin, lid and hose connections,
B
7 = plastic mesh plant racks, 8 = 6 mm PVC rod frame, 9 = 25 mm garden trellis mesh, 10 = plastic cable ties used in construction and to attach plants, 11 = collection details written on waterproof paper with marker pen
|
Water for the cascade was drawn from a depth of 5 m about 100 m offshore from the laboratory on the southern side of Cape Cleveland, near Townsville (19o14' S: 147o03' E) and passed through two 1-megalitre settling tanks with a residence time of about 24 h. Water temperature in the cascade varied between about 20o and 31oC, remaining within 2 oC of inshore surface seawater temperature throughout the year. During the frequent windy periods the water was still turbid when it reached the cascade. Particularly rapid growth of Halimeda plants often occurred then, probably because of the increase in dissolved nutrients which Walker and O'Donnell (1981) showed to occur when the sediment was disturbed in this area. Plants were examined for gametangia at approximately daily intervals and any accumulation of silt on the plants was removed at that time by energetic dousing of the racks in the seawater. Plants normally remained free of visible epibiota and did not require cleaning except when occasionally fouled by sponge growth or mass settlement of ascidians.
An individual collection was deemed to be fertile on those days on which gametangia were observed to have developed on one or more plant fragments in that collection. Fertile plants from the cascade were preserved in 4% formaldehyde in seawater for later examination. To allow as much time for gametangial expansion as possible, preservation was usually delayed until about 1600 h. Fertile material was obtained opportunistically in the field, either by Scuba divers or from grab samples at various localities throughout the GBR, and preserved in the same way. Microscope preparations of gametangial clusters were obtained by shaving them off the surface of segments with a sharp blade. This avoided the necessity for decalcification and possible changes in dimensions which may have resulted from chemical and osmotic activity. Excision close to the segment surface could usually be confirmed by the shape of the base of the gametophores. Loss of cytoplasm through the cut end, another possible cause of change in gametangial dimensions, appeared minimal, presumably because of cytoplasmic plugs already developed within the gametangial cluster. Material was drawn at about 100 x magnification either with an optical drawing tube or by tracing onto clear acetate sheets the direct or recorded image from a JVC GX-N7E colour video camera attached to a compound microscope.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
RESULTS Veg growth & repro-
Sexual reproduction-
Seasonality-
Structure-
Release of gametes
Results
Vegetative growth and reproduction
Data set out in Table 1 show the Halimeda species included in this study, the taxonomic authorities, and the degree of success in growing them in the cascade. Particularly luxuriant growth of new segments was observed in plants of H. tuna, H. discoidea, H. opuntia, H. hederacea, H. distorta and H. melanesica and H. spp. Most other species grew sporadically. In some species, such as H. lacunalis and H. hederacea, there was a lag of several weeks before new growth occurred. Plants of H. macrophysa and H. micronesica did grow new segments but seldom survived more than a few weeks in the cascade whilst those of H. fragilis did not grow at all on the two occasions they were introduced.
Table 1. Growth and development of iHalimeda species in the cascade
|
| Species |
Growtha |
Fertilityb |
|
|
Monthc |
|
|
Field |
Lab |
Stressd |
JFMAMJJASOND |
|
| Halimeda |
| H. discoidea Decaisne |
***R |
4 |
27 |
0 |
JF________SOND |
| H. gigas W.R.Taylor |
* |
1 |
1 |
0 |
____________ND |
| H. gracilis Harvey ex J.Agardh |
*** |
0 |
5 |
0 |
J____________D |
| H. lacunalis W.R.Taylor |
** |
0 |
2 |
2 |
_F____________ |
| H. macrophysa Askensay |
* |
0 |
5 |
0 |
____________ND |
| H. taenicola W.R.Taylor |
NA |
3 |
0 |
0 |
J______________ |
| H. tuna (Ellis & Solander) Lamouroux |
*** |
1 |
38 |
4 |
JF_______ASOND |
|
| Opuntia |
| H. copiosa Goreau & Graham |
*** |
4 |
1 |
1 |
__M_M___A____ |
| H. spp |
*** |
4 |
1 |
1 |
JF____________D |
| H. distorta (Yamada) Colinvaux |
*** |
0 |
10 |
0 |
J____________ND |
| H. hederacea (Barton) Hillis |
*** |
6 |
14 |
1 |
JFMA__J_____ND |
| H. opuntia(L.) Lamouroux |
*** |
2 |
12 |
0 |
_FM_MJ___SO__ |
_
|
| Micronesicae |
| H. fragilis W.R.Taylor |
-- |
2 |
0 |
0 |
____________N_ |
| H. melanesica Valet |
***R |
0 |
24 |
0 |
JFM_______OND |
| H. micronesica Yamada |
* R |
3 |
5 |
0 |
_F__M____S_ND |
|
| Rhipsalis |
| H. cylindracea Decaisne |
** |
0 |
0 |
1 |
__________Z___ |
| H. incrassata (Ellis) Lamouroux |
***R |
3 |
4 |
1 |
J___________ND |
| H. macroloba Decaisne |
***R |
0 |
5 |
0 |
JF__________ND |
|
| Total |
|
29 |
162 |
11 |
|
|
a Growth; -- = none, * = Poor, ** = moderate, *** = good, R = runners formed, NA = not attempted
b Fertility; initials of months = fertile, _ = not fertile, Z = stressed only
c Results combined for the 6-year period
d Possible stress during transport or in cascade - not used in seasonality analysis
|
|
In addition to growth of new segments, a number of species established discrete new plants on the racks at the end of fine rhizoids. This occurred most frequently with H. macroloba, H. discoidea and H. melanesica, and once with H.micronesica and H.incrassata. A time series showing the rate of new segment development and instances of rhizoid development in H. discoidea is set out in Fig. 3. The new thalli had a small bulbous holdfast of uncorticated filaments below the basal segment. Discrete new plants with characteristic basal structures have also been observed to proliferate from segments of some moribund plants, particularly in H. macroloba and H. melanesica.

Fig. 3. Segment, rhizoid and new plant growth in H. discoidea in the cascade.
Light green segments had grown since previous date; red segments on baseline indicate those lost from positions marked by arrows since previous date, day 0 = June 29, day 106 = October 13
RESULTS Veg growth & repro-
Sexual reproduction-
Seasonality-
Structure-
Release of gametes
Sexual reproduction
Maintenance of considerable amounts of several species of Halimeda in the cascade provided the opportunity to observe the striking events of its sexual reproduction. Although plants of H. tuna and H. discoidea very occasionally initiated development of the transparent gametophore axis prior to nightfall, development of gametangial structures usually occurred completely in the dark and the first indication of fertility was parts of plants, whole plants or groups of plants covered with stalked clusters of green gametangia when first observed in the morning, having been indistinguishable from normal plants the day before. The underlying segments were by then very pale or pure white and the subsequent course of events usually followed the time course described by Hillis-Colinvaux (1980) with the gametangia becoming progressively darker throughout the day and, if left overnight, being colourless the next day. On several occasions fertile plants of H. discoidea were transferred to aerated tubes in a controlled environment room overnight. After a 12 h dark period, the plants were re-illuminated with artificial light at sunrise (0600 h) the next day and by the time they were first observed at 0800 h the water was a green, or in some instances golden brown soup of gametes. Plants grown in the cascade thus behaved normally throughout the sexual process.
RESULTS Veg growth & repro-
Sexual reproduction-
Seasonality-
Structure-
Release of gametes
Seasonality of gametangia formation
The months when gametangia were observed on the thalli of 17 species of Halimeda are set out in Table 1 together with the number of times each species was found fertile either in the field or in the cascade. Of the 202 fertility events observed, it was considered possible that 11 of those in the cascade were triggered by unnatural stresses such as traces of hydrocarbon contamination in the seawater, fungal infection, or unfavourable conditions during transport from the reef. These stress-related occurrences of gametangia have been omitted from further considerations which are therefore based on 191 events in a total of 17 species. Of those species, most have been identified according to Hillis-Colinvaux (1980), but one (H.hederacea) has been resurrected, for reasons set out by Drew and Abel (1987), from a description by Hillis-Colinvaux (1968), and another is as yet unidentified and will be called H. spp. The latter has considerable affinity with H. hederacea but it has smaller segments with a distinctly crenullate upper margin, is usually restricted to very shallow situations and is particularly tolerant of high water temperatures.
Data set out in Fig.4A show that fertility of Halimeda plants from the GBR was concentrated in the austral spring and summer months. Our observation show that gametangia formation in the seawater cascade peaked in plants from the central section in September, with a secondary peak in February, whereas the peak for those from the northern and southern sections was several months later, around mid-summer. Data in Fig. 4 B and C show that these differences were not due to the fertility of different numbers of species in these months, since the number of species forming gametangia peaked in summer in material from both the central and the other sections of the GBR. Rather, as shown in Fig. 4 D, the differences could be traced to the behaviour of the two most prolifically fertile species. H. tuna and H. discoidea. Together these two species accounted for 35% of all the fertility events observed, and 82% of those which occurred in September and October. Plants of those two species from the southern and northern sections were fertile considerably later than those from the central section.

|
Fig. 4. Seasonality of Halimeda gametangia formation in the Great Barrier Reef
A number of times gametangia found per month (all species, n = 191),
B total number of species with gametangia each month,
C as B but central, southern and northern sections separated,
D number of times gametangia found per month on individual species for which n > = 10.
Months arranged to place austral midsummer in centre (Hatched bars = northern section, filled bars = central section, open bars = southern section)
|
Data in Table 1 and Fig. 4 D also show that the fertility of many species was restricted to only a few months of the year, particularly if only material from either the central or the other sections is considered. Furthermore, for the central section the amount of data available is sufficient to indicate that these periods were staggered between early summer (H. tuna, H. discoidea), midsummer (H. melanesica, H. distorta) and late summer (H. hederacea). Other species such as H. copiosa, H. opuntia, and H. micronesica showed no such seasonality of fertility. The restriction of fertility to particular times of year is further supported by data in Fig. 5 which shows the periods for which collections of the more prolifically fertile species were grown in the cascade, together with the times of gametangia formation. Most of these plants had been growing in the cascade for a number of months prior to the formation of gametangia so that influences from their original habitat were unlikely to have persisted, especially as the thalli then often consisted either entirely or mostly of segments which had grown in the cascade.
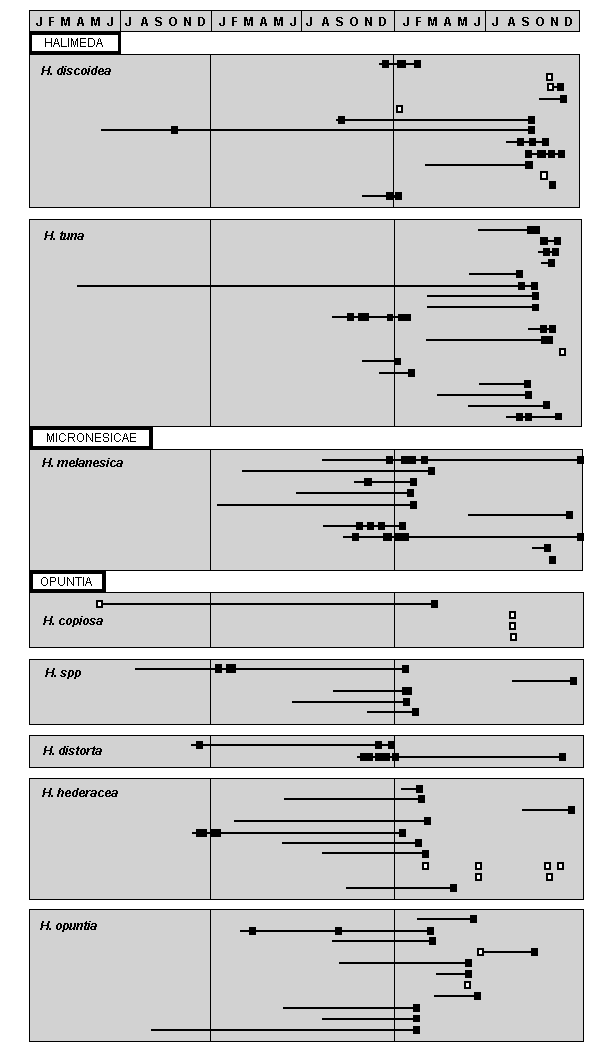
Fig. 5. Seasonality and occurrence of repeated gametangia formation in collections of seven species of Halimeda from the Great Barrier Reef
lines = duration of growth of a single collection in cascade, filled squares = days on which gametangia observed in the field. The 3-year sequence represents the maximum duration of growth of a collection in the cascade, not any particular calender years
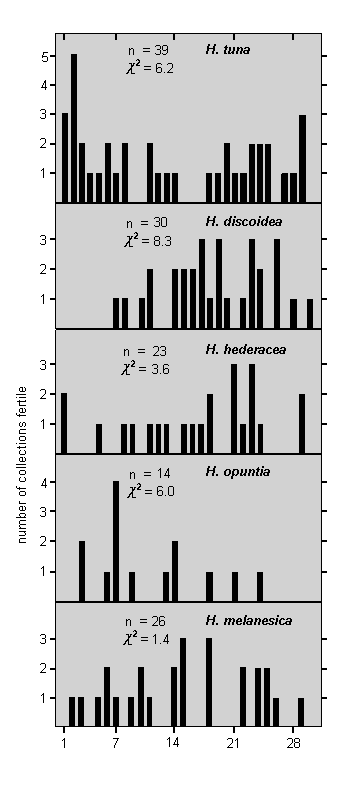
|
Fig. 6. Lunar periodicity of fertility
X2 calculated on hypothesis that fertility events would be evenly distributed throughout months if no lunar periodicity occurred)
|
Some plants in collections of H. copiosa, H. discoidea, H. distorta, H. hederacea, H. melanesica and H. spp. remained vegetative during the first occurrence of fertility in other plants from those collections. Some of these subsequently became fertile about 12 months later (24 months for H. tuna) with no occurrences of fertility observed for those collections in the interim period. In contrast, different plants from a single collection of H. opuntia, a species which showed no regularity in the timing of fertility, became fertile three times at intervals of only six months in the cascade.
Lunar periodicity in gametangia formation was sought by allocating each fertility event to the appropriate day of the lunar cycle, and then calculating chi-squared values on the hypothesis that, in the absence of periodicity, equal numbers of events should occur in each quarter of the month. Analysis of all 191 instances of fertility together indicated no lunar periodicity at all. However, data set out in Fig. 6 for individual analysis of each species which formed gametangia on at least 14 different occasions indicate that fertility in H. discoidea was significantly correlated with lunar periodicity at the 5% level of probability, whilst in H. tuna and H. hederacea the correlation was possibly significant at the 10% level. H. melanesica and H. opuntia showed no correlation, although the number of events recorded for the latter may be too small to be certain of this. Fertility appears to be correlated with different quarters of the lunar cycle in different species, a feature which would mask any widespread lunar periodicity in the combined analysis of all fertility events.
RESULTS Veg growth & repro-
Sexual reproduction-
Seasonality-
Structure-
Release of gametes
Structure of gametangial clusters
Typical gametangial clusters of 12 of the species observed fertile are shown in Fig. 7. These are all drawn to the same scale and include H. distorta, H. fragilis, H. gigas, H. hederacea, H. melanesica and H. taenicola for which gametangia have not been reported before. Gametangia were regularly arranged around the distal part of gametophores which were usually dichotomously branched at least once and had a basal, gametangia-free portion of considerable length. The diameters of gametangia differed considerably between species, ranging from 250-300 um in H. melanesica and H. opuntia to about 100 um H. discoidea, H. distorta, H. fragilis, H. macrophysa and H. taenicola. Quantitative measurements of various characteristics of the gametangial clusters of 7 species are set out in Table 2.

|
Fig. 7. Typical gametangial clusters of twelve species of Halimeda, all drawn at the same scale.
A Section Opuntia, B section Micronesicae, C section Halimeda.
Gametangial clusters have not been described previously for H. distorta, H.fragilis, H. gigas, H. hederacea,H. melanesica and H. taenicola
|
Table 2. Quantitative data for gametangial characteristics.
Values in parentheses=1 standard deviation. n = number of gametangia measured, G/C = number of gametangia per cluster, P/C = number of papillae per cluster, G/P = number of gametangia per papilla
|
| Species |
Gametangia |
|
|
|
|
Gametophore |
|
|
Diam (um) |
n |
G/C |
P/C |
G/P |
width (um) |
length (um) |
|
| H. discoidea |
104.6 <15.9) |
149 |
11.5 |
2.9 |
4.0 |
40.4 (8.1) |
1055.4 (175.7) |
| H. tuna |
138.2 (19.1) |
87 |
3.6 |
1.1 |
3.4 |
36.3 (4.8) |
595.0 (112.2) |
| H. copiosa |
130.2 (25.2) |
48 |
6.9 |
2.0 |
3.4 |
41.0 (6.5) |
824.1 (69.2) |
| H. hederacea |
147.0 (26.3) |
201 |
8.7 |
1.8 |
4.9 |
36.9 (4.9) |
1128.5 (282.7) |
| H. opuntia |
211.1 (45.8) |
268 |
7.9 |
2.5 |
3.2 |
45.1 (7.8) |
999.2 (113.2) |
| H. melanesica |
234.1 (46.2) |
33 |
6.6 |
2.0 |
3.3 |
50.0 (4.1) |
1052.8 (263.8) |
|
| H. micronesica |
123.5 (21.9) |
29 |
14.5 |
3.5 |
4.1 |
60.0 |
964.0 |
|
In all species, the gametophore branches ended in club shaped tips which had a thickened cap, and several such tips usually projected slightly beyond each gametangial cluster. In the species with larger gametangia these tips were also larger. In all 7 species examined quantitatively, there was one tip for every 3 or 4 gametangia. The role of these structures as discharge papillae will be described below.
RESULTS Veg growth & repro-
Sexual reproduction-
Seasonality-
Structure-
Release of gametes
Release of gametes
Various stages in the release of gametes from H. melanesica are shown in Fig. 8. Immediately prior to the start of release from a gametangial cluster, the coloured protoplasmic contents were confined to the upper portion of the gametangia which appeared dark green whilst the remainder of the structure was transparent. Gamete release was initiated when this dense mass in one gametangium dissociated rapidly into many discrete motile units and expanded downwards through the gametangial stalk. A nearby unexpanded tip then ruptured just below the thickened cap, allowing release of the contents under pressure as a mucilaginous strand of discrete gametes. This process was repeated in other gametangia in rapid succession, accompanied by the rupture of one more tip in the cluster. The contents of the 13 gametangia in the cluster illustrated were emptied through only two tips in less than 20 min, leaving only a few discrete gametes inside and several other tips un-ruptured. Release of gametes from the whole plant was completed within one hour.
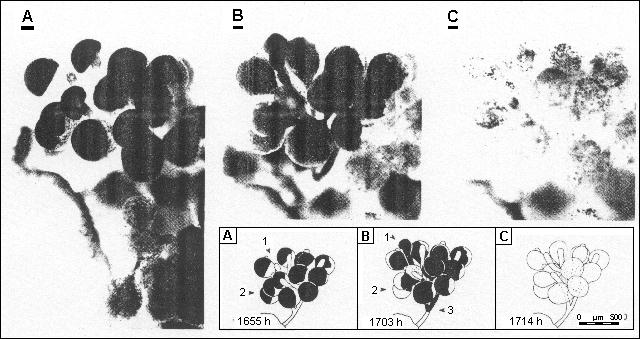
|
Fig. 8. Stages in the discharge of gametes by H. melanesica
Numbered arrows in inset indicate: 1 = discharge papillum involved, 2 =gametes initially confined to upper part of gametangium then moving downwards, 3 = flow of gametes down gametophore branches diverted upwards towards discharge papillum at the lowest bifurcation
|
The original slides for this sequence are currently unavailable
Click here to see an alternative sequence for H. tuna
|
The details illustrated were observed when a plant unexpectedly released gametes in the late afternoon of the day on which the gametangia were first observed. Such release at about 1630 h has since occurred several times with H. melanesica in the cascade and also with H. incrassata found fertile in the field. In all the other species observed, gamete release has occurred soon after dawn the next day, just as described by Hillis-Colinvaux (1980). Occasionally some clusters did not release their contents within the first hour after dawn, in which case the gametes were never released actively and presumably decayed with the rest of the plant.
Gamete release could be delayed by keeping fertile plants in running seawater in the dark until sometime between sunrise and at least noon the next day. Gamete release then commenced between 5 and 15 min after re-illumination. Those species having larger gametangia, such as H. opuntia, responded more slowly and also had less explosive gamete release processes. Gamete release also appeared to be less vigorous the longer this was artificially delayed after true dawn. This procedure has been used in our laboratory both to obtain fresh gametes at convenient times of day for mating and fine-structure studies (A. Siotas, personal communication) and also to obtain video recordings of the process of gamete release. Preliminary analysis of these recordings has revealed a number of features of the process. The discharge papillae swelled slightly prior to explosive rupture around the base of the distinctly thickened cap which usualIy remained partially attached. Discharge of gametes was preceeded by a substantial quantity of white or pale brown mucilaginous material which could be seen in some cases floating upwards or sinking away from copiously discharging plant as numerous small globules visible to the unaided eye. The dark green mass inside a gametangium became transformed into a mass of active particles only seconds before release commenced, significant displacement of cytoplasmic material within gametophores occurred immediately before the explosive rupture of the cap of the discharge papilla, and the contents of different gametangia in a single cluster could be released either as streams of discrete gametes or as several large packets which remained intact even outside the plant. The size of the gametangia often decreased significantly during the first few seconds of discharge, but remained constant thereafter.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Discussion
Thirteen of the 17 Halimeda species tested grew well from portions of plants several segment long suspended from plastic mesh racks in flowing seawater at ambient inshore water temperature and 20% ambient sunlight. Prolific growth of new segments in the cascade and subsequent subdivision of the plants often resulted in plants consisting entirely of segments grown therein. Plants of several species also reproduced vegetatively by establishment of new plants attached to the plastic mesh racks at the ends of fine rhizoids. Macroscopic characters such as segment shape were well maintained in all new growth and microscopic features such as utricle dimensions and arrangement remained characteristic of the species involved even though material from disparate habitats was being grown under virtually identical conditions. This is probably the nearest approach to experimental taxonomy which has yet been applied to Halimeda.
After several years of opportunistic observation and collection of fertile Halimeda plants from the cascade and in the field, a marked seasonality became apparent. Several species became predictably fertile during a species-specific period of 2-3 months varying from early spring (September) to late summer (February). This is contrary to the suggestion by Hillis (1959) and Hillis-Colinvaux (1980) that plants became fertile throughout the year. However, her detailed observations were for only one species (H. incrassata), did not appear to cover more than about six months and were made under laboratory controlled conditions wherein plants would not receive seasonal cues from the environment and would therefore be unlikely to demonstrate any seasonality. We have found several species bearing gametangia in the field at similar times of year to those in the cascade, and in one instance (H. hederacea) even on the same day. It therefore seems likely that events in the cascade are representative of the situation on at least the nearby reefs, and any seasonality observed in the cascade may also be expected to occur in the field where similar environmental conditions prevail.
The 17 species observed in the cascade exhibited considerable variation in the reproductive strategies adopted. Gametangia production was much more prolific in species such as H. discoidea, H. melanesica and H. tuna than in the sprawling species which are extremely successful at vegetative reproduction. Nearly all plants of the former species kept in the cascade eventually produced gametangia whereas only about 20% of the large number of H. hederacea and H. opuntia plants kept therein became fertile. Our observations have also led us to conclude that H. micronesica is an opportunisitic species. It rarely grew even a few new segments in the cascade but the presence of conspicuous dead plants of this species at all times of year at any site where it grows, and the lack of any seasonality in its production of gametangia are consistent with a rapid turnover and predominantly sexual reproduction. H. cylindracea grew well in the cascade but did not produce gametangia, except once in response to hydrocarbon contamination. However, that species frequently died back to just a few segments from which new plants subsequently grew, a process suggestive of a perennation mechanism.
The substantial body of data presented for the large segmented species H. tuna and H. discoidea demonstrates an unequivocal seasonality, at least at the latitude of Townsville which is at 19o S and therefore experiences considerable meteorological seasonality. Data are set out in Fig. 9 for the annual variation of inshore seawater temperature, daylength and daily irradiance which both the cascade and local field material would have experienced. Irradiance is unlikely to be limiting at any time of the year and, as the range of the irradiance-related variables is relatively small, it is difficult to correlate them with times of fertility. On the other hand, we have found Halimeda metabolism particularly sensitive to temperature with, for instance, marked reduction of carbon fixation by most species when seawater temperature reaches 29 to 30 oC (Abel and Drew 1985, and unpublished data). There may be a correlation between fertility and seawater temperature through spring and summer. H. tuna, the first species to become fertile in 4 successive summers, may be responding to seawater temperature increase after the annual low in early August. Fertility of this species seems to coincide with a water temperature of about 26 oC. H. discoidea has always followed very soon after, then H. melanesica and H. distorta, whilst H. hederacea and H. spp. may be responding to the high temperatures, up to 31 oC, which occur soon after midsummer. Such suggestions are amenable to experimental verification, particularly in view of the ease of culture of markedly seasonal species such as H. tuna and H. discoidea.
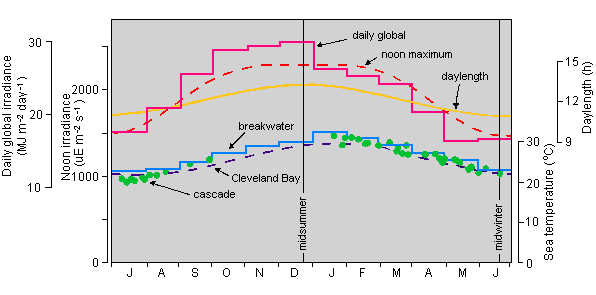
|
Fig. 9. Environmental factors affecting the seawater cascade.
Light. Daylength from Smithsonian Meteorological Tables (List 1958). Daily global irradiance = monthly means (380-3000 nm) from August 1982 to August 1984, measured at Townsville with an integrating solarimeter. Noon irradiance = maximum clear sky PAR (photosynthetically active radiation, 400-700 nm) computed from astronomical relationships) NB seawater cascade received only 20% of full daylight.
Temperature. Filled circles = spot measurements in cascade, solid stepped line = monthly means at Townsville breakwater (Kenny 1974), broken curve = Cleveland Bay (near AIMS laboratory, Townsville) computed using formula of Walker (1981), T=4.05 sin (0.01717 x (D + 72.2)) + 25.4 where t = oC, D = day of year
|
Beth (1962) reported that the appearance of fertile plants of H. tuna in the Mediterranean Sea began in June but reached a peak in late August/early September. This is at the end of summer in that locality when the sea, although never reaching tropical temperatures, is warmest, and that author suggested a temperature of at least 18 oC is required for fertility, which is considerably lower than the 26 oC which appears to required by that species in the GBR. Chihara (1956) also suggested that increased water temperature may have been the trigger for gametangia formation in H. cuneata in Japan. Reported times of fertility of various Halimeda species in various parts of the world are set out in Table 3. Some of these differ markedly from our observations, in some cases even for areas as close as New Caledonia, and for species such as H. discoidea which are distinctly seasonal on the GBR. It therefore seems likely that local seasonal factors such as temperature and nutrient supply may indeed be more important than global factors such as daylength and season, whilst the fact that not all plants of even the most prolifically fertile species became fertile during their first year in the cascade indicates interaction between external stimuli and some internal factors in the initiation of gametangia. The importance of purely local factors is also supported by the considerable delay in formation of gametangia observed in the H. tuna and H. discoidea material transported to Townsville from localities several hundred kilometres to the north and the south. Fertile plants of H.discoidea were present in the field at the northern localities at the same time as material from the central section reefs was fertile in the cascade. Thus, a second fertility period for these plants may have been induced by the stress of midsummer cascade seawater temperature above that which they may have experienced in their natural environments, or perhaps the move and new conditions caused changes in metabolism which interrupted the accumulation of compounds required to initiate gametangia production. Certainly, seawater temperatures at the more southerly Heron Island reef are lower overall but the stress hypothesis is not supported by the continued vegetative growth of some H. tuna from there through two consecutive summers in the cascade, whilst the northern plants, when observed fertile in the field in November, were already experiencing temperatures of 27 to 28 oC, similar to those in the cascade at that time.
Table 3. Reports of Halimeda fertility from other regions (compiled from the literature).
Locality codes plus references subsequent to Hillis (1959):
A= Antilles (Lesser and Greater), B = Bahamas, F = Florida, G = Guam (Merten 1971), H= Hawaiian Islands, I = Indonesia (Hillis-Colinvaux 1972), J = Japan, M = Mediterranean (Beth 1962), N = New Caledonia (Garrigue 1985), 0 = Okinawa (Kamura 1966), P = Phillipines, R = Puerto Rico, Z = Great Barrier Reef (Saenger 1979).
Na, Wa = fertile in unheated aquaria in New Caledonia (Garrigue 1985) and Western Australia (L. Marsh personal communication) respectively
|
| Species |
J | F | M | A |
M | J | J | A |
S | O | N | D |
Halimeda |
| H. cuneata |
. | . | . | . | Wa | J | J |
J | . | . | . | . |
| H. discoidea |
. | R | N | . | . | . | . |
. | . | . | H | . |
| H. macrophysa |
. | . | . | I | . | . | I |
. | . | . | . | . |
| H. scabra |
. | . | F | . | . | . | F |
. | . | . | . | . |
| H. tuna |
. | . | PR | . | . | M | M |
M | M | M | M | M |
Opuntia |
| H. opuntia |
. | . | . | . | . | . | IOP |
O | OP | . | . | . |
Micronesicae |
| H. micronesica |
. | . | . | . | . | . | I |
. | . | . | . | . |
Rhipsalis |
| H. cylindracea |
. | . | . | . | . | . | . |
. | . | Na | Z | . |
| H. favulosa |
. | B | . | . | . | . | . |
. | . | . | . | . |
| H. incrassata |
N | . | NR | A | F | FO | OPR |
O | . | . | B | BN |
| H. macroloba |
GN | G | GN | G | GP | . | I |
G | G | G | G | GN |
| H. monile |
. | . | . | . | . | . | . | . |
. | . | . | A |
| H. simulans |
. | . | . | . | . | . | F | . |
. | . | . | . |
|
Effects of a further meteorological factor, lunar periodicity, was also detectable in some of our observations of Halimeda fertility, including H. tuna. Beth (1962) detected a rather imprecise 28 day periodicity in the formation of gametangia in H. tuna in the Mediterranean and he suggested this may be linked with the lunar cycle. However, chi-squared analysis of his 96 observations over two years revealed no significant difference at all in the frequency of gametangia formation in the 4 quarters of the lunar month.
The understanding of any marked seasonality in Halimeda fertility and resulting death of the fertile plants is important in the context of sediment dynamics in areas where vast deposits of dead segments have accumulated beneath luxuriant meadows of living Halimeda, such as those behind the outer ribbon reefs of the northern G13R (Drew and Abel 1985, 1988). Mass disintegration of sexually spent plants may have been occurring there regularly for several thousand years at the same time of year. We have recently observed the simultaneous fertility of up to 25% of the many thousands of H. discoidea plants visible to a diver in two such meadows 100 km apart on the same day. This occurred in early November when collections of H. discoidea in the cascade would be expected to become fertile repeatedly over a period of several weeks, suggesting that more of the vegetative material still remaining in the meadows could have become fertile soon after our observations.
The availability of fertile Halimeda in the laboratory has allowed us to make a number of observations concerning the structure and function of the gametangia themselves. Species-specific differences in the size of gametangia, which ranged from about 100 um to over 250 um, were statistically significant and were consistent in fertile material obtained from several different collections from different reefs and in successive years. However, they were not correlated with the section of the genus to which the species are assigned, there being a wide range of sizes in at least the sections Opuntia, Micronesicae and Halimeda. The diameter of gametangia was observed to decrease significantly as internal pressure was explosively relieved during the first few seconds of gamete relese. However, it is unlikely that gametangia would have been stretched by this pressure when material was preserved the day before as it would then have to be maintained for more than 12 h. It is also unlikely that the size differences are due to the formation of separate gametangia containing either macro-or micro-gametes, particularly as there is no indication of such differentiation in the literature and we have now observed in both H. discoidea and H. tuna that both types of gametes can arise from gametangia of similar size. The larger gametangia, and possibly also the wider discharge papillae of species such as H. opuntia and H. melanesica may therefore be useful taxonomic characters, as Hillis-Colinvaux (1980) originally suggested but had insufficient data to substantiate. Such characters may be particularly useful in the difficult Opuntia section of the genus, and have already provided part of the evidence supporting the resurrection of the species H. hederacea. A more complete analysis of the considerable amount of preserved material now available will be the subject of a later publication.
The occurrence in all species of several unexpanded gametophore tips in each gametangial cluster was established and their role as discharge papillae (Hillis-Colinvaux 1980) clearly substantiated. Considerable pressure was clearly involved in the first few moments of gamete release, firstly in rupturing the top of the discharge papilla around the cap and then in explosively forcing the mucilage and gametes out. A lesser, sustained pressure then continued to force gametes and mucilage out for many minutes thereafter. Our observations on the structures and events associated with gamete release in Halimeda also conform closely with those described and illustrated for the closely related Chlorodesmis bulbosa by Ducker (1965). The structure and function of the apical discharge tubes (our papillae) were especially similar.
The extension of the siphons to form gametangial structures during the night in Halimeda is akin to the night-time development of the medullary filaments of its new segments, a process which we have recorded using time-lapse photography. It is also probable that the intriguing cellular mechanism which allows rapid overnight movement of the entire protoplasmic contents of each segment into superficial gametangia and initially confines the pre-gamete mass to the top of the gametangium is related to the regular mass migration of the chloroplasts within vegetative Halimeda segments. For reasons as yet undetermined, the chloroplasts move from the surface utricles into the medullary filaments during the first few hours after dark and then begin to move out again several hours before dawn (Abel and Drew 1983). This process occurs every night and, if the other colourless organelles move at the same time, their subsequent accumulation in tubes projecting beyond the utricles would be readily explained.
GO TO Title-
Abstract-
Methods-
Discussion-
Results-
Discussion-
References
References
- Abel KM, Drew EA (1983)
- Chloroplast movement in Halimeda.
Aust Soc Plant Physiol Conf (abstr)
- Abel KM, Drew EA (1985)
- Response of Halimeda metabolism to various environmental parameters.
Proc 5th Int Coral Reef Symp 5:21-26
- Beth K (1962)
- Reproductive phases in populations of Halimeda tuna in the Bay of Naples.
Publ Sta Zool di Napoli, Italy 32:515-534
- Chihara M (1956)
- Studies on the life-history of the green algae in the warm seas around Japan. IV. On the life history of Halimeda cuneata Hering.
J Japan Bot 31:201-210
- Derbes AA, Solier JJ (1956)
- Memoire sur quelques points de la physiologie des algues.
C R Acad Sci 1: 1-20
- Drew EA (1983)
- Halimeda biomass, growth rates and sediment generation on reefs of the Central Great Barrier Reef Province.
Coral Reefs 2: 101 -110
- Drew EA, Abel KM (1985)
- Biology, sedimentology and geography of the vast inter-reefal Halimeda meadows within the Great Barrier Reef Province.
Proc 5th Int Coral Reef Symp 5:15-20
- Drew EA, Abel KM (1988)
- Studies on Halimeda. I. The distribution and species composition of Halimeda meadows throughout the Great Barrier Reef Province.
Coral Reefs 6:195-205
- Ducker SC (196.5)
- The structure and reproduction of the green alga Chlorodesmis bulbosa.
Phycologia 4:149-162
- Garrigue C (1985)
- Repartition et production organique et minerale de macrophytes benthiques du Lagon de Nouvelle-Caledonie.
PhD thesis, Universite des Sciences et Techniques du Languedoc, Montpelier
- Hillis L (1959)
- A revision of the genus Halimeda (order Siphonales).
Publ Inst Mar Sci University of Texas 6:321-403
- Hillis-Colinvaux L (1968)
- New species of Halimeda: a taxonomic reappraisal.
J Phycol 4:30-35
- Hillis-Colinvaux L (1972)
- Reproduction in the calcareous green algae of coral reefs.
J Mar Biol Ass India 14:328-334
- Hillis-Colinvaux L (1980)
- Ecology and taxonomy of Halimeda: primary producer of coral reefs.
Adv Mar Biol 17:1-327
- Hillis-Colinvaux L (1984)
- Systematics of the Siphonales.
In: Irvine DEG, John DM (eds) Systematics of the green algae. Academic Press, London Orlando
- Kamura S (1966)
- On the sexual reproduction of two species of Halimeda (Chloropyta).
Bull Arts and Science, University of the Ryukyus, Math Nat Sci 9:302-313
- Kenny R (1974)
- Inshore sea surface temperatures at Townsville.
Aust J Mar Freshwater Res 25:1-5
- List RJ (1958)
- Smithsonian meteorological tables, 6th rev edn.
Smithsonian Inst Washington. Misc Coll 114:506-507
- Meinesz A (1972)
- Sur le cycle de l'Halimeda tuna (Ellis & Solander) Lamouroux (Udoteacee, Caulerpale).
C R Acad Sci ser D 275: 1363-1365
- Merten MJ (1971)
- Ecological observations on Halimeda macroloba Decaisne (Chlorophyta) on Guam.
Micronesica 71:27-44
- Milliman JD (1974)
- Recent sedimentary carbonates, pt 1: Marine carbonates.
Springer, Berlin Heidelberg New York
- Nasr AH (1947)
- Synopsis of the marine algae of the Egyptian Red Sea coast.
Bull Fac Sci 26:1-155
- Saenger P (1979)
- Records of subtidal algae from the Swains Reef Complex, Great Barrier Reef, Queensland.
Proc R Soc Queens 90:51-55
- Walker TA (1981)
- Annual temperature cycle in Cleveland Bay, Great Barrier Reef Province.
Aust J Mar Freshwater Res 32:987-991
- Walker TA, O'Donnell G (1981)
- Observations on nitrate, phosphate and silicate in Cleveland Bay, Northern Queensland.
Aust J Mar Freshwater Res 32:877-887
- Williams GC (1975)
- Sex and evolution.
Princeton University Press, Princeton, NJ









