Please note that this material is copyright to the publishers of Botanica Marina and to the authors
Not to be reproduced in any way without permission except for personal study

Botanica Marina, Vol 33: 31-45, 1990
Studies on Halimeda III.
A Daily Cycle of Chloroplast Migration within Segments*
Edward A. Drew and Kay M. Abel
Australian Institute of Marine Science, PMB No. 3, M.S.O., Townsville, Queensland 4810, Australia
*Contribution No. 473 from the Australian Institute of Marine Science
(Accepted 8 July 1989)
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Abstract
Under natural conditions of irradiance, daylength and water temperature, the segments of Halimeda plants rapidly become very pale after dark, remain so for most of the night, but are again green enough by dawn to permit rapid photosynthesis as soon as light is available. This diurnal paling phenomenon affects the entire plant and has been observed in the field, in a laboratory seawater cascade system and in laboratory experiments under controlled conditions (25oC, 350 uEm-1 s-1, 12 h light/12 h dark). Plants also pale dramatically when placed in the dark at any time of day. These changes in surface pigmentation are not due to changes in chlorophyll concentration, which remains above 45 ug Chl cm-2 throughout the day and night. However, microscopic examination showed that dramatic migrations of chloroplasts occur within segments, whilst timelapse photography has allowed quantification of the resulting changes in surface pigmentation. Immediately after dark, chloroplasts begin to migrate from the surface utricles, through the secondary utricles, and into the internal medullary filaments which are beneath the calcium carbonate exo-skeleton. This movement is reversed before dawn the next day, suggesting that, although the inward migration of the chloroplasts may be directly triggered by the onset of darkness, initiation of movement in the opposite direction is not directly controlled by light.
More than a hundred chloroplasts 3 to 3.5 um wide must pass through the 5 to 6 um wide constrictions at the base of each surface utricle during only about one hour after dark, so chloroplast migration must be highly organised to avoid blocking these narrow openings. The chloroplasts were associated with, and appeared to move along a network of cytoplasmic striations which are probably bundles of microtubules. Several of these striations pass into each surface utricle through its basal constriction, thus providing a structural basis for the rapid, co-ordinated movement of so many organelles.
The phenomenon of chloroplast withdrawal from the plant surface, a feature necessarily restricted to coenocytic algae without crosswalls, is discussed in the context of observations in related genera, its relationship with large scale re-arrangement of organelles during sexual reproduction in Halimeda, and its possible roles both in minimising damage from surface grazers and in the rapid development of new segments.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Introduction
Occasional reference has been made in the literature to the fact that Halimeda plants may become pale during experimental manipulations, particularly in the dark (Borowitzka and Larkum 1976, Stark et al 1969). Those workers did not perceive this as a major problem in their work. However, the paling of Halimeda plants seriously interferred with our experiments on the light saturation characteristics of Halimeda photosynthesis. During those experiments it was necessary to expose plants to various irradiance levels for at least 30 minutes each, including darkness for measurement of respiration. During the dark period, the plants invariably became paler irrespective of the time of day. They recovered their original greenness only slowly during subsequent periods in the light and during that time had reduced photosynthetic rates. They also became pale and photosynthesised slower in the late afternoon and evening even without any dark periods, seriously limiting the time available to obtain reproducible photosynthetic measurements. We also noted that Halimeda plants in our aquaria became pale at night.
Having concluded that this fluctuation in surface pigmentation was a natural diurnal occurrence rather than an artefact of our experiments, we set about establishing how this phenomenon progressed. In particular, we sought any less dramatic fluctuations which might occur during the daytime period, and also investigated the response of plants to different irradiance levels and water temperatures, both variables in our photosynthesis experiments. In this paper we shall show that the paling of Halimeda plants is due to actual movement of chloroplasts into the interior of the segments, describe the diurnal pattern of this movement both in the field and also in the laboratory under irradiance and temperature conditions similar to those experienced in the field, and we shall discuss possible mechanisms and consequences of this phenomenon.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Methods
Plant material
For the laboratory studies, an opuntioid species of Halimeda was used which has not yet been fully identified. It was the same as that referred to as Halimeda spp. by Drew and Abel (1988), has small, crenellated segments and grows extremely well on reef flats, and also in the seawater cascade in which it was cultured in the laboratory. Plant stocks were originally collected from a fringing-reef flat at Cockle Bay, Magnetic Island, near Townsville. It proved particularly suitable for these experiments, being quite heavily calcified and so provided considerable contrast between the dark green day-time condition and the almost white night-time condition when the chloroplasts were hidden beneath the calcium carbonate exoskeleton. This species, together with H. hederacea (Barton) Hillis and H. opuntia (L.) Lamouroux., were also studied in the field.
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Anatomical observations
Material preserved at different times of day and night was obtained by incubating branches in aerated seawater in glass tubes kept at 25 oC in a water bath. Illumination at 100 uEm-2 s-1 was provided by white fluorescent tubes which were switched off at 18:00 h and on again at 06:00 h (12 h light/l2 h dark). Formalin was added to different tubes at various times by an automated system of syringes activated under the control of an Epson HX20 microcomputer. The final formaldehyde concentration in the tubes was 4% in seawater. Segments of preserved branches were decalcified in dilute HCl (20%) prior to microscopy which was carried out on an Olympus BH2 microscope with Nomarski interference contrast illumination. Microphotographs were taken using Kodak Panatomic-X film.
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Time-lapse photography
a. Field observations
These were carried out at Davies Reef, near Townsville, in the central section of the Great Barrier Reef Province. Underwater photographs were obtained either by SCUBA divers. with a Nikonos underwater camera, close-up attachment, electronic flash and Kodak Ektachrome 400 slide film, or by deploying a Hanimex Amphibian underwater camera with built-in electronic flash and motor film-drive, a close-up lens, an automated triggering device and Kodacolor 100 colour negative film. The same patch of Halimeda opuntia was photographed by the divers both in the afternoon and then again at 21:00 h, several hours after dark. Overnight sequences of underwater photographs of a patch of H. spp. and another of H. hederacea were obtained with the automated system which also had a waterproof digital watch in the field of view for accurate timing.
b. Laboratory experiments
Experimental material consisted of apical branches, 5 to 7 segments long. The branches were severed from the parent plant by ligation with a noose of nylon thread pulled tight around a node between two segments. The noose was held tight for 1 minute to allow at least preliminary wound-sealing reactions to occur before severance was completed. Three such branches were suspended between fine nylon threads supported by a vertical perspex frame to maintain the orientation of the segments to the camera whilst allowing uninterupted water flow around them. Some paling of the segments occurred as a result of the physical damage of ligation, so the branches were usually set in place and allowed to recover for several hours in 0.45 um filtered seawater (FSW) at 25 oC and illuminated at 350 uEm-2 s-1. Experiments were then started with no further manipulation of the plant material, thus avoiding even the slightest physical shock which might have stimulated anomalous chloroplast movement.
The experiments were carried out in a light-tight room. Irradiance during pre-treatment and experiments was provided by 2 Hanimex 2555 tungsten halide slide projectors. A domestic time-switch turned the projectors off at about 18:00 h and on again at 06:00 h, producing alternating light-dark cycles of approximately 12 h duration which were synchronised with natural daylight (see Results for exact durations used). The frame supporting the experimental material was immersed in a 12-litre flat-sided glass incubation tank which contained FSW and was itself immersed in distilled water in a glass-sided controlled mixing temperature bath at 25oC � 0.2oC. A Nikon 35 mm camera fitted with electronic flash, motor drive, and film-back to hold 250 frame cassettes was used to record changes in the surface pigmentation of the Halimeda segments as changes in the optical density of their negative images on Kodak Panatomic-X black and white film. The interval between photographs was controlled by an external timing circuit and was either 12 or 90 minutes. In some experiments, a secondary timer prevented any photography for several hours each night. The exact time was recorded in each film frame by means of a digital watch in the camera's field of view, and the output from a silicon photo-voltaic cell was recorded on a chart recorder to monitor both light projector output and the occurrence of photographic flashes.
Rates of photosynthesis and respiration were measured in a second incubation chamber, immersed in the same controlled temperature bath and also in the camera's field of view, which held a single Halimeda branch mounted in a frame similar to that in the main incubation tank. This smaller cylindrical glass chamber contained 135 ml of FSW, and was sealed with a rubber bung which held a probe to measure dissolved oxygen (Yellow Springs Instruments) connected to a chart recorder. A magnetic stirrer bar, rotated at constant speed by a Rank submersible magnetic stirrer situated below the chamber, ensured adequate of the incubation medium. Values of percent oxygen saturation and the output of the silicon photovoltaic cell were digitised and stored by a Campbell CR21 data logger and transferred daily to tape via an Epson HX20 microcomputer.
The experiments lasted for between 2 and 6 days, and the FSW in both chambers was changed, usually daily, to reduce nutrient limitations. A slow stream of air was introduced into the main incubation chamber in order to generate some water movement whilst not disturbing the orientation of the Halimeda segments relative to the camera. The complete experimental system is shown diagramatically in Figure 1 and typical day and night frames in Figure 2.
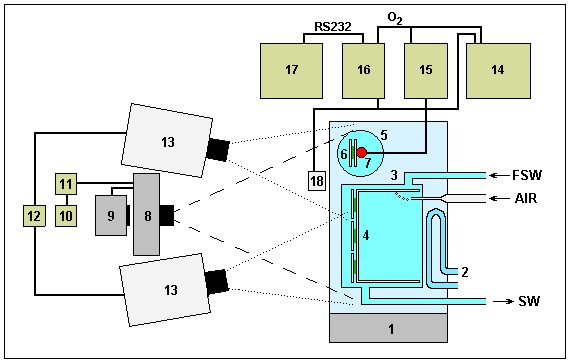 |
Fig. 1. Laboratory incubation and time-lapse photography system.
|

|
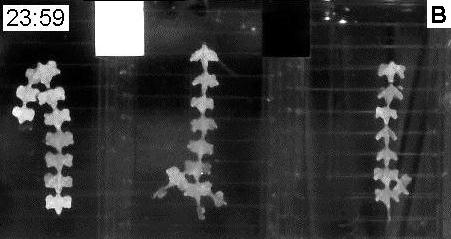
|
|
Fig. 2. A. Day (11:59 am) and B. night (11:59 pm) frames from a time-lapse film (Film A1)
|
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Analysis of laboratory time-lapse films
Films were processed using standard photographic procedures and then the density of the images of individual segments on consecutive frames were analysed using a macro-densitometer system This consisted of an Elmo S-300 filmm-strip projector and a screen of white mellanite-covered chipboard with a hole in the centre housing a LICOR quantum detector with the upper surface of its opal sensor flush with the screen surface. The photographic images were projected onto the screen at a constant magnification such that a single Halimeda segment completely covered the sensor. The sensor was connected to a LICOR LI 188-B Integrating Quantum Radiometer and a WESLOG datalogger (connected to the recorder output of the radiometer) functioned as an A-D converter, sending its output to the RS232 port of an IBM Portable computer. Software written in BASIC allowed appropriate data files to be created on floppy diskettes and also provided for subsequent analysis. Several variables could have interfered with the accuracy of the analysis process, including film processing variability along a film and between films, variation in electronic flash intensity, and fluctuations in light output from the bulb of the film-strip projector. These were corrected for by the inclusion and measurement of standard black and white surfaces in each film frame. The light intensity at the screen for a given segment was then expressed as a percentage of the difference between the readings for the black and white surfaces. As the film contained the negative image, these values represent the degree of blackness of the original scene and may be directly equated with the quantity of pigment in the surface utricles, which we have called surface pigmentation.
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Segment area
The surface area of growing segments was measured on the time-lapse films using a computer-video system which allowed the video image of a film frame to be combined with the video output of a Commodore Amiga 1000 computer via a Genlock interface. Mouse-driven software written in BASIC allowed the outline of segments to be drawn on screen and the enclosed area to be calculated directly.
METHODS
Plant material -
Anatomical observations -
Time-lapse photography -
Film analysis -
Segment area -
Chlorophyll
Pigment analysis
The chlorophyll content of freeze-dried Halimeda branches was determined by spectrophotometry of acetone extracts. The equations of Jeffrey (1968) were used to calculate the chlorophyll a and b contents, and the surface areas of the segments making up the branches were measured to 0.1 cm2 using a LICOR LI 3000 area meter with an LI 3050 conveyor system. This system was also used to measure the surface area of segments used in photosynthesis/respiration experiments, and both pigment content and oxygen metabolism are expressed per unit area of segment.
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Results
Diurnal surface pigmentation under natural conditions
Field observations were made on Halimeda growing at 6 to 8 m depth on lagoon patch reefs. In an initial series of afternoon and evening photographs taken by SCUBA divers, all the segments of a substantial patch of H. opuntia were bright green in the afternoon but were very pale by 21:00 h, only 3 h after dark. In situ time-lapse photography of a patch of Halimeda spp., the same species used in laboratory experiments, showed that paling of the segments commenced soon after dark, progressed to its maximum extent well before midnight, and began to be reversed about 2 h before dawn. The magnitude of paling in these colour photographs was dramatic and it happened in all visible segments. Less dramatic but still quite noticable paling was also observed in a series of time-lapse photographs of H. hederacea.
In addition to these opuntioid species, night-time paling has also been observed in H. cylindracea Decaisne, H. discoidea Decaisne, H. distorta (Yamada) Colinvaux, H. lacunalis W. R. Taylor and H. tuna (Ellis et Solander) Lamouroux. Surface pigmentation changes were dramatic in all except H. cylindracea which still showed detectable differences between night and day.
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Quantitative analysis of diurnal surface pigmentation changes
The data set out in Figure 3 show the diurnal changes in surface pigmentation of Halimeda segments during one light/dark cycle in the laboratory. Values are plotted at 12 minute intervals for one segment on each of 4 separate branches. A very rapid decrease in pigmentation began less than an hour after dark, and the decrease was more or less complete by 2 hours after dark. Surface pigmentation then remained at a constant low level for several hours until a few hours before dawn when it began to increase, although only at about one third the rate of the preceding decrease. Initiation of the pre-dawn increase occurred at differ�ent times in different segments but was always more than half completed by dawn, and completed about 2 hours thereafter, Also shown in Figure 3 are the rates of net photosynthetic oxygen production in the light and respiratory oxygen consumption in the dark by one of the branches. Rates of respiration were highest immediate after darkness began and reduced progressively during the night, most rapidly during the first half. Rates of photosynthesis at least as high as those the previous afternoon occurred as soon as the lights were switched on again, rose only slightly during the period that surface pigmentation continued to increase to the day-time maximum and then began to decrease slowly.

|
Fig. 3. Diurnal changes in surface pigmentation and oxygen exchange in laboratory time-lapse system.
Experiment begun on 20 March, Halimeda branches in place by 14:00 h, temperature 25 oC, irradiance 350 uEm-2 s-1 with 11.75 h dark period. Plants photographed and surface pigmentation determined at 12 min intervals, segments analysed are shown cross-hatched in branch diagrams with corresponding numbers against graphs. Light/dark cycle indicated by open (light) and filled (dark) bars on time axis. Thick line represents data from Halimeda branch in oxygen measuring chamber and lower graph shows rates of change of dissolved oxygen calculated at 2 min intervals subjected to Fourrier smoothing (Film A6).
|
Two consecutive diurnal sequences for another four branches are shown in Figure 4. These were also photographed at 12 minute intervals but frames were analysed at only hourly intervals. As in the previous experiment, the segments analysed on the different branches behaved very similarly throughout, although in this experiment they were initially pale, not having had long enough to recover from the trauma of setting up before photography began. Nevertheless, they still showed further paling which commenced soon after dark, and the pre-dawn increase in surface pigmentation began about 3 hours before dawn, progressing at about a quarter of the preceding rate of paling. Full day-time surface pigmentation was then maintained until the start of the next dark period. Data on photosynthetic oxygen exchange are not available for this experiment. Similar diurnal patterns of change in surface pigmentation were shown by the branches in 3 other experiments carried out at various times of year, with the pre-dawn increase in pigmentation beginning between 2 and 4 hours before dawn in all experiments.
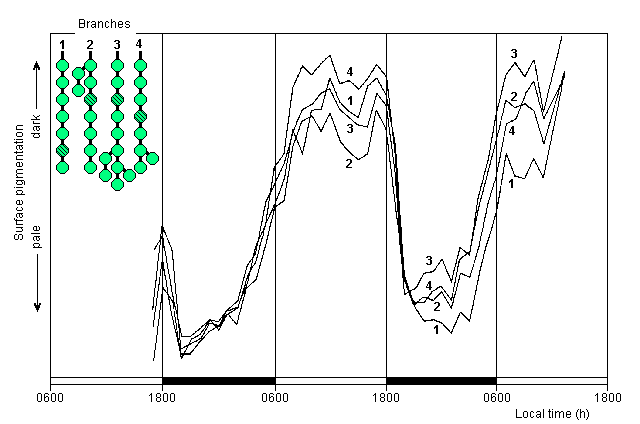
|
Fig. 4. Diurnal changes in surface pigmentation over two-day period.
Experiment begun 13 February, Halimeda branches in place by 16:30 h, and not fully recovered from sampling trauma, temperature 25 oC, irradiance 400 uEm-2 s-1 with 12 h light/12 h dark cycle, incubation water changed at noon on second day. Plants photographed at 12 minutes intervals but only every fifth frame analysed (1 hour intervals). Other details as in Figure 3 (Film A6).
|
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Lack of effect of light flashes during the night
The data in Figures 3 and 4 were obtained from timelapse photographs exposed with an intense electronic flash of about 1 millisecond duration every 12 minutes. Data are set out in Figure 5 for a 6 day experiment conducted to determine the effect, if any, of these frequent light flashes during the dark period on the diurnal cycle of surface pigmentation changes. During the first day, photographs taken every 12 minutes established the same diurnal pattern found in the previous experiments. During the second night no photographs were taken from the start of the dark period until midnight, but subsequent exposures at 90 minute intervals showed that the minimum surface pigmentation for the night had still been achieved by midnight and the subsequent pigmentation increase followed the expected pattern. During each of the next 4 nights only 4 flash photographs were taken, at 19:00 h to check initiation of the post-darkness decrease, 20:30 h to check progress towards minimum surface pigmentation, 04:00 h to check maintenance of the low levels for most of the night, and 05:30 h to check the degree of pre-dawn pigmentation increase. Even under these minimal flash conditions, the essential features of the diurnal cycle of pigmentation established during the first 24 h were accurately maintained for the subsequent 5 days and nights including the initiation of increase in surface pigmentation several hours before dawn. The progressively decreasing amplitude of surface pigmentation changes may have been due to the fact that the incubation FSW was not changed after the second day, probably resulting in depletion of some essential nutrients.

|
Fig. 5. Diurnal changes in surface pigmentation over 6 days with minimum flash frequency during darkness.
Experiment begun 12 April, Halimeda branches in place previous day, temperature 25 oC, irradiance 350 uEm-2 s-1 with 12 h light/12 h dark cycle. Plants photographed every 12 minutes on day 1 but only every fifth frame analysed, flashes reduced to 90 min interval on night 2 with none between 18:15 and 23:45 h (= broken line on graph), then to only 4 (19:00, 20:30, 04:00 and 05:30) from night 3 to 6. Other details as in Figure 3 (Film A10).
|
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Chlorophyll content throughout the day
Data are shown in Table I for the chlorophyll content of apical branches of Halimeda spp at various times during the diurnal cycle. Pigment concentrations remained relatively unchanged during the large fluctuations in surface pigmentation which were visually detected in this experiment as well as in all those documented above.
|
Table 1. Diurnal variation in chlorophyll concentration in Halimeda spp.
|
|
| Time1 |
Chlorophyll a + b (ug Chl cm-2) |
|
| 12:03 h |
46.0 |
| 18:03 h |
50.3 |
| 21:03 h |
69.6 |
| 24:00 h |
50.0 |
03:39 h |
49.8 |
| 06:10 h |
50.7 |
| 09:17 h |
72.9 |
| 12:02 h |
45.3 |
|
| mean |
54.3 +/- 3.8 (standard error) |
|
|
1 Plants in darkness between 18:00 h and 06:00 h
|
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Direct observation of chloroplast distribution within segments
The macroscopic appearance of Halimeda segments fixed in formalin at various times of day and night, and then decalcified, are shown in Figure 6. These surface views with transmitted light show the progressive accumulation of pigmentation in the thickened ribs and towards the base of each segment.
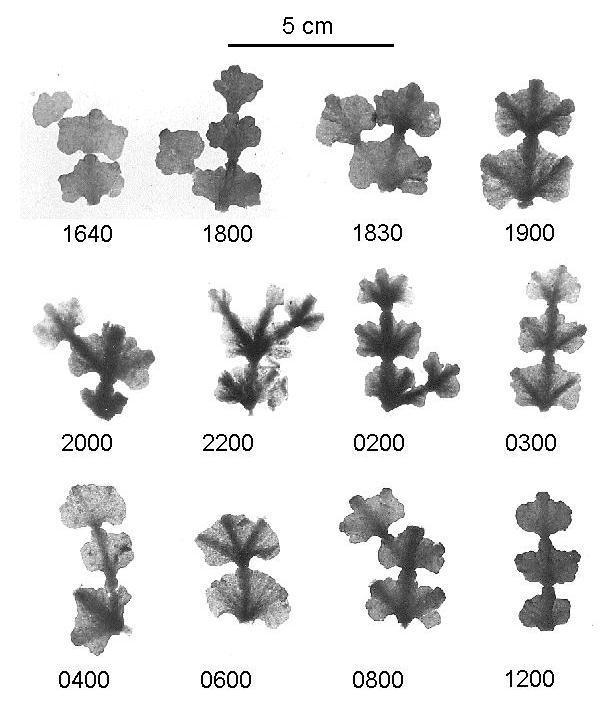
|
Fig. 6. Diurnal sequence of decalcified segments of Halimeda spp. showing congregation of pigmented material.
(Photographed by transmitted light).
|
Microscopic examination of surfaces and hand-cut sections of decalcified segments showed that the changes in pigment distribution within the segments were closely correlated with large scale redistribution of chloroplasts, as illustrated in Figure 7A, B (day) and C, D (night). During the day large numbers of chloroplasts were closely packed against the upper surface of each of the surface utricles. These organelles measured 6 to 7 x 3 to 3.5 um, so there was enough space for about 80 packed against the upper surface of the utricles which had a mean surface area of about 1700 um2. As there appeared to be at least two layers in each utricle, these must each contain at least 160 chloroplasts. In the day-time sections there were only a few chloroplasts in the secondary utricles and medullary filaments, and very few structures of any kind were visible therein except for occasional clusters of clear globules 9 to 13 um in diameter. These globules correspond to amorphous structures seen in electron micrographs of Halimeda. They seem to form the basis of wound-response plugs seen by light microscopy at the ends of broken filaments (Drew and Sturmey, unpublished observations) as has also been described in several other genera of siphonous algae [see Menzel (1988) for review].
The microscopic appearance of the surfaces and sections changed dramatically after dark, with movement of chloroplasts out of the surface utricles being well advanced only 30 minutes after the lights were turned off and virtually complete after 2 hours. The surface, or primary utricles were shaped like inverted cones with an internal diameter of only about 6 um at their junction with the underlying secondary utricies. Chloroplasts were observed singly, or very occasionally two at a time, in these constrictions, and always seemed to have been travelling with their long axis parallel to that of the opening.
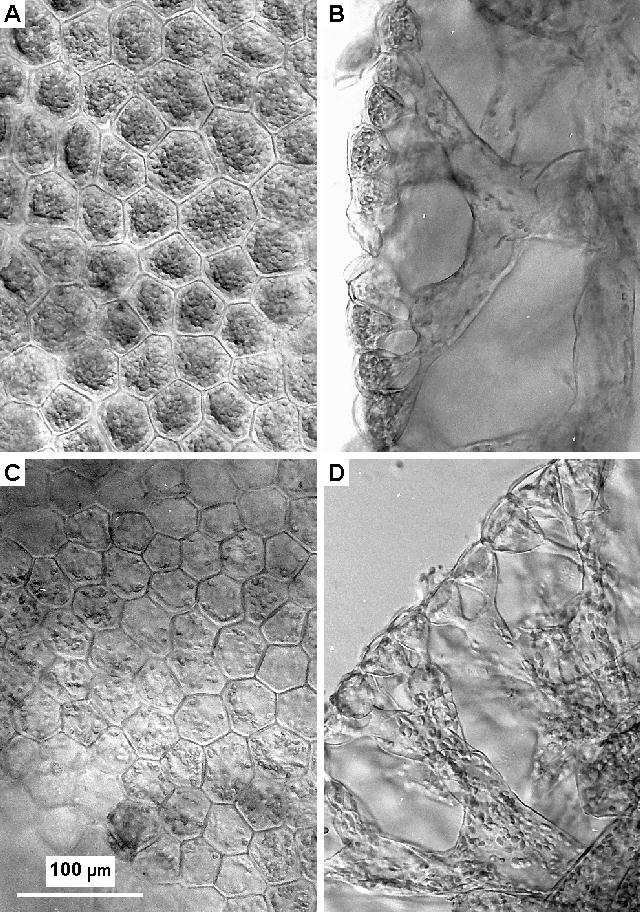
|
Fig. 7. Distribution of chloroplasts within segments during day and night.
Daytime material preserved at 16:39 h, nighttime at 22:00 h, both in 4% formaldehyde/seawater. A - daytime surface view of surface utricles; B - daytime cross-section showing surface (primary) and secondary utricles and medullary filaments; C - nighttime surface view; D - nighttime cross-section.
|
An extensive network of fine branching striations was visible under Nomarski illumination in many of the filaments, particularly in the larger medullary filaments, nodes and apical tufts sometimes observed extending beyond the calcified matrix of the segments at specific points on the segment margin where new segments might otherwise have been expected to develop. Such striations are illustrated in Figure 8A, B, and the chloroplasts seemed to be aligned along them. Preliminary observations of live material also showed the chloroplasts to be moving along visible striations. Several of these striations can be seen passing through the basal constrictions of the surface utricles (Figure 8C) whilst the star-like structures visible in surface utricles (Figure 8D) in both day and night samples appear to be the combined terminations of these.
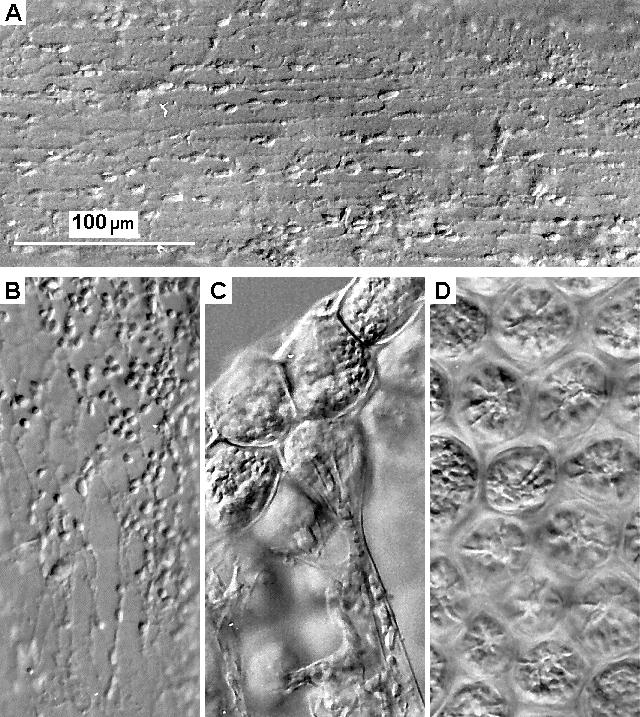
|
Fig. 8. Details of cytoplasmic striations in both filaments and utricles.
Plant material preserved in 4% formaldehyde/seawater and viewed at x 400 magnification under Nomanski illumination. A - filament in apical tuft; B - filament in region of node between segments; C - Surface utricle - side view, daytime; D - Surface utricle - underside, daytime.
|
RESULTS
Diurnal colour changes -
Qantitative analysis -
Effect light flashes -
Chlorophyll -
Chloroplast distribution -
Growth new segments
Growth and pigmentation of new segments
In two experiments several new segments developed during the time-lapse sequences. Detailed analysis of changes in surface area and surface pigmentation in one of these are set out in Figure 9. Results from the other experiment followed the same pattern. Segments increased in area only during the first 24 hours of development. Expansion was approximately exponential throughout that period, including the night, until a few hours after dawn when it suddenly ceased completely. Surface pigmentation of the new segments remained very low from inception to a few hours before dawn on their second day. At that time, which coincided exactly with the re-emergence of chloroplasts in the old segments, the new segments began to become pigmented and by the time they ceased areal expansion, they were very much more highly pigmented than their parent segments. The parent segments were themselves less pigmented than they had been the day before or became the following day. Surface pigmentation of the new segments then began to decrease rapidly after their expansion ceased and by 18:00 h (dusk) their pigmentation was similar to that of their parent segments.

|
|
Fig. 9. Surface pigmentation and areal expansion of new segments and their parent segment.
|
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
Discussion
Halimeda plants become pale in colour in the dark both during the normal day/night diurnal cycle and also during experimental treatments. This has also been reported by Stark et al. (1969) and Borowitzka and Larkum (1976) who both noted its adverse effect on photosynthetic, and possibly also calcification rates, and attributed the phenomenon, without supporting evidence, to movement of the chloroplasts away from the periphery of the segments. We have confirmed these substantial changes in surface pigmentation and have been able to demonstrate, by chemical analysis and microscopic examination, that the paling of segments was indeed a result of dramatic redistribution of chloroplasts into the interior of the segments rather than any change in chlorophyll content. Such long range redistribution of organelles is only possible because of the coenocytic nature of the Halimeda thallus and the marked colour change results from the accumulation of the chloroplasts below the opaque calcium carbonate exo-skeleton which surrounds the primary and secondary utricles. In all the experiments in which the changes in surface pigmentation have been quantified from time-lapse photographs, paling of the segments was detectable about 30 minutes after dark, and microscopic examination showed that by this time many of the chloroplasts had already moved out of the surface utricles into the underlying secondary utricles but were presumably not yet concealed beneath the exo-skeleton. Initiation of chloroplast movement was therefore probably a direct and immediate response to the onset of darkness. However, re-emergence of chloroplasts the next morning began well before dawn and therefore appears to be part of an intrinsic rhythm and certainly not a response to re-illumination. The attaining of a constant, low level of surface pigmentation early in the night resulted from all mobile chloroplasts passing beneath the opaque exo-skeleton and does not preclude further redistribution having occurred out of sight, although pigment accumulation into the thickened ribs and segment bases also seemed to reach a steady state well before midnight, with a densely packed layer of chloroplasts probably lining the medullary filaments for most of the night. This regular and dramatic change in colour of Halimeda plants in the field may have escaped the notice of marine botanists because the phenomenon is not noticable until about an hour after dark and is almost completely reversed by dawn, a period of time when such workers do not usually observe algae!
The re-emergence of chloroplasts well before dawn was not an artefact of the frequent intense light flashes associated with the photographic procedure, since the reduction of night time flashes from 59 to only 4 had no effect. This is in agreement with the report by Zurzycki et al. (1983) that flashes up to 10 times as long as those used in our experiments had no effect on chloroplast re-orientation in Lemna. Re-emergence did proceed considerably slower than the post-dark�ness withdrawal and despite its early start, did not result in all chloroplasts being at the segment surface by dawn. However, there were enough there to allow rapid photosynthesis to commence as soon as radiant energy became available, a feature of some ecological importance in the field where, at several metres depth, irradiance increases only slowly up to saturation level over a period of an hour or more after first light. In the dark, the rate of oxygen consumption was initially very high, but this dropped rapidly during the first few hours of the night, and may therefore have been a result of the considerable energy demand for rapid movement of large numbers of chloroplasts. However, the rate continued to drop, albeit more slowly, until dawn, including several hours during which large scale chloroplast movement had recommenced, suggesting that the energy demand of chloroplast movement alone was below the detection level of these respiration measurement. The initial high rate of dark respiration, and its subsequent decline, must have been due to other causes, perhaps rapid utilisation and depletion of photosynthetically generated respiratory substrates, or adverse effects of the reduction of dissolved oxygen in the incubation chamber to a minimum of 65% saturation before dawn.
The mechanical organisation of these chloroplast migrations must be efficient since a large number of substantial organelles pass through the constricted bases of the surface utricles and then continue to move rapidly into and along the medullary filaments for distances up to 104 um in the larger segmented species. In the species studied in detail, at least 160 chloroplasts, each 6 to 7 um long, must pass one at a time through the narrow base of each surface utricle before reaching the 50 to 70 um wide secondary utricles and medullary filaments. Since all the chloroplasts pass through the basal constrictions within one hour, they must move at a minimum velocity of 17 um min-1. Other organelles such as mitochondria and nuclei may be moving at the same time, making the potential for congestion even greater.
Chloroplast movement has been studied in many multicellular plants (see review by Haupt 1982) and in general the response to darkness is for the chloroplasts to be arranged in a randomly spaced layer along those call walls which were, and presumably will be the next day, perpendicular to the light. This is the same arrangment as occurs under moderate levels of irradiance, and occurs in pseudoparenchymatous algae as well as higher plants. Unlike the situation in Halimeda, which appears to initiate chloroplast movements in response to darkness, their rapid reorientation in most plants is a response to particularly bright light, when they become aggregated in self-shading arrays against the cell walls parallel to the light. Nultsch et al. (1981) concluded that, at least in Dictyota, Alaria and U1va, the function of such reorientation was not the direct regulation of photosynthetic activity but rather protection from photo-damage. Drew (1979) also concluded that considerable paling of the leaves of the seagrass Halophila stipulacea (Forsk.) Aschers. in bright light was due to chloroplast movement to form clumps, rather than to loss of chlorophyll. Since the inhibition of photosynthesis which occurred initially at high irradiance was completely alleviated after clumping, despite the extremely pale colour of the leaves which resulted, he too concluded that movement of the chloroplasts resulted in reduction of photo-damage.
The distance over which most plants can move their chloroplasts is necessarily limited by the dimensions of the cells. However, studies have also been carried out on the long distance movements which occur in several coenocytic algae. Studies on Vaucheria (Fischer-Arnold 1963, Blatt et al. 1981, Blatt 1983) have concentrated on the spectral sensitivity of movement which was found to occur in response to blue, but not red, light. Such considerations have little relevence to the dark response observed in Halimeda, but a number of other studies on caulerpalean algae closely related to Halimeda are very relevant. Koop et al. (1978) described a cyclic migration of chloroplasts between apex and basal rhizoids in Acetabularia which occured even in continuous light, and then as a circadian rhythm with a period of 25 to 27 h. The basipetal component of this migration was correlated with times of darkness, as were similar movements described by Dawes and Barilotti (1969) in Caulerpa. Close similarities therefore exist between these algae and Halimeda since, if the surface utricles of the latter are considered multiple apices, chloroplast reorientation at night results in an apical portion devoid of chloroplasts in all 3 genera. Studies of fine structural aspects of chloroplast movement along microtubules and actin filaments, recently reviewed by Williamson (1986), have shown this to be clearly related to the more general phenomenon of cytoplasmic streaming. Although these studies were initially confined to the giant cells of characean algae (Kamiya 1986), they have recently been extended to Vaucheria by Blatt et al. (1980) and also to algae more closely related to Halimeda. Particularly significant are fine structural studies on Chlorodesmis and physiological studies on Bryopsis. Menzel (1985) described the close alignment of plastids along microtubule bundles in Chlorodesmis. The concentric lamellar system, a structure unique to caulerpalean chloroplasts and called a thylakoid organising body in Halimeda by Borowitzka and Larkum (1974), always pointed in the direction of travel and Menzel postulated that it provided the energy for movement. Menzel and Schliwa (1986 a, b), using immunochemical techniques, showed that Bryopsis also has an extensive and complex cytoskeleton of microtubules and actin filaments along which chloroplasts move. That movement was inhibited or severely disorganised by a wide range of compounds known to be inhibitors of processes connected with microtubules and actin fibres. Similarly extensive microtubule arrays have been demonstrated ultrastructurally in Udotea by Mariani-Colombo (1978).
The rates of chloroplast movement found in Bryopsis by Menzel and Schliwa (1986 a) were up to 60 um min-1, but more usually about 30 um min-1, whilst mitochondria moved at up to 120 um min-1 along a different channel system with which the nuclei also seemed to be associated. Such rates of chloroplast movement are similar to the minimum calculated for Halimeda. A network of striations bearing a striking resemblance to the microtubule/actin filament network described in Bryopsis was also observed in Halimeda (using Nomarski illumination), despite the very simple fixation procedure we used, and we have observed chloroplasts moving rapidly along such structures in live preparations. Thus, the chloroplast transport mechanism in the two genera may well have the same structural basis, whilst the continuity of several striations through the constricted bases of the surface utricles in Halimeda, and their radial array within those utricles terminating at a central point, may represent a very high level of functional microtubule organisation ideally suited to the coordinated and rapid transport of many large organelles through narrow openings.
The energy expended in maintaining and operating such a dynamic cyto-skeleton must be considerable but the several benefits to the plant which are immediately apparent probably justify such expenditure. The benefits include rapid reproductive development, controlling grazing damage, and the rapid achievement of autonomy by new segments. Sexual reproduction in Halimeda is preceded by redistribution of all organelles within the segment into superficial gametangial clusters where they remain for a whole day before release as gametes at dawn the next day. A long distance organelle transport system is clearly required for this, and as most of the movement related to reproduction takes place between dusk and dawn 2 nights before release, it is reasonable to hypothesize that the chloroplasts and other organelles are merely diverted beyond the surface utricles during their normal movement from the interior of the segments a few hours before dawn. The mechanism of chloroplast withdrawal must then be deactivated or physically blocked to allow them to remain in the gametangia for the whole night prior to discharge. However, this process occurs only once in the life of a Halimeda plant, death resulting directly from the holocarpic release of the entire cytoplasmic contents as gametes, making this unrealistic as a complete explanation for a daily mass migration of chloroplasts throughout the life of the plant. A second, probably more important result of this chloroplast migration which may be useful every night is the absence of vital organelles from the plant surface during the night when nocturnal grazers are active. Although this will be of little significance when grazers such as fish take large bites from segments, such predation on Halimeda is deterred to a considerable extent by the high concentrations of diterpenoid icthyotoxins such as the halimedatrial present therein (Paul and Fenical 1983, 1984). On the other hand the ravages of surface scrapers are frequently visible as thin white lines meandering all over the surface of segments, and we ascribe much of that type of damage to a small, bright green, sacoglossan mollusc which may be attempting to harvest Halimeda chloroplasts for its own use in much the same way as Elysia viridis does from Codium (Trench et al. 1973) and Tridachia crispata and Tridachiella diomedea do from Caulerpa (Trench et al. 1972). Thus, Halimeda has the ability to avoid such chloroplast loss by moving them beneath the calcium carbonate exo-skeleton at night when they serve no purpose at the segment surface anyway, and redistributing them to undamaged surface utricles the next morning. This may be a unique defensive mechanism, or it may be another useful side-effect of a cytoplasmic streaming mechanism with an even more fundamental purpose, the rapid greening of new segments.
New segments develop directly on older segments in Halimeda, connected by several wide filaments, and they grow to maximum size within 24 hours with most growth occurring during the night. Our observation that surface pigmentation began to increase rapidly in the dark several hours before dawn, despite the established inability of green plants to synthesise more than traces of chlorophyll in the dark, suggests strongly that the new segments were initially populated with chloroplasts from their parent segments. There was also a slight reduction in surface pigmentation of the parent segments on the day when the new segments became green, as compared with the days immediately before and after. Although those differences were small and their statistical significance cannot yet be demonstrated because new segments were such a rare occurence in our experiments, the changes observed may nevertheless have resulted from movement of chloroplasts out of the parent segments and their subsequent replacement de novo. This greening during darkness is in agreement with the remarks of Borowitzka and Larkum (1974) that new segments of H. cylindracea usually became green in the dark, although those authors did not consider the possible origin of chloroplasts in their new segments by any means other than development from proplastids in situ. In our experiments, the new segments were very highly pigmented by the time areal expansion ceased soon after dawn, and the progressive decrease in pigmentation observed throughout the rest of that day was probably due to changes in the optical properties of the segment surface as the calcium carbonate exoskeleton developed, rather than to any reduction of chlorophyll content. Rapid calcification would be particularly important as Paul and Alstyne (1988) showed that new, uncalcified segments are initially protected from grazers by large amounts of a very toxic compound - halimedatrial - but this is soon replaced by smaller amounts of a less toxic compound halimedatetracetate. Hay et al. (1988) were able to show that segments of Halimeda less than one day old were consumed in preference to older segments by small parrotfish and that new segments were produced in the dark, even when day and night were artificially reversed. They considered this nocturnal growth pattern to be an adaptation to diurnal variation in herbivore activity, which was minimal at night whilst the new segments were most nutritious but least defended by calcification. Those authors did not consider the actual source of photosynthetic pigmentation in the new segments but hypothesised that greening was delayed until just before dawn to minimise the risk of losing valuable molecules until they could be of direct use in the light. Since calcification in Halimeda is greatly stimulated by photosynthesis, the migration of a large population of mature chloroplasts from the calcified parent segments into the new segments shortly before dawn would effectively minimise such risks whilst ensuring that calcification for long-term defence began at the earliest possible time.
Such advantages accruing from the rapid redistribution of chloroplasts, and possible other organelles as well, are only available to coenocytic algae. It is possible that the considerable success of the siphonous green algae in dominating the macro-algal flora of highly grazed tropical corals reefs is dependent in part upon these effective mechanisms which minimise the exposure of chloroplasts, new segments and reproductive structures to risk.
Acknowledgements
We would like to acknowledge the contributions of Helen Sturmey, who analysed the pigments and did the photomicrography, Les Brady who processed the many time-lapse films, and Mike Cuthill who prepared the photographic illustrations
GO TO Title-
Abstract-
Introduction-
Methods-
Results-
Discussion-
References
References
- Blatt, M., N. Wessels and W. Briggs. 1980.
- Actin and cortical fibre reticulation in the siphonaceous alga Vaucheria sessilis.
Planta 147: 363-375.
- Blatt, M., M. Weisenseel and W. Haupt. 1981.
- A light-dependent current associated with chloroplast aggregation in Vaucheria sessilis.
Planta 152: 513 - 526.
- Blatt, M. 1983.
- The action spectrum for chloroplast movement and evidence for blue-light-photoreceptor cycling in the alga Vaucheria.
Planta 159: 267 - 276.
- Borowitzka, M. A. and A. W. D. Larkum. 1974.
- Chloroplast development in the caulerpalean alga Halimeda.
Protoplasma 81: 131 - 144.
- Borowitzka, M. A. and A. W. D. Larkum. 1976.
- Calcification in the green alga Halimeda. IV. The action of metabolic inhibitors on photosynthesis and calcification.
J. Exp. Bot. 27: 894 - 907,
- Dawes, C. J. and D. C. Barilotti. 1969.
- Cytoplasmic organisation and rhythmic streaming in growing blades of Caulerpa prolifera.
Am. J. Bot. 56: 8 - 15.
- Drew, E. A. 1979.
- Physiological aspects of primary production in seagrasses.
Aquat. Bot. 7: 139 - 150.
- Drew, E. A. and K. M. Abel. 1988.
- Studies on Halimeda. II. Reproduction, particularly the seasonality of gametangia formation, in a number of species from the Great Barrier Reef Province.
Coral Reefs 6: 207 - 218.
- Fischer-Arnold, G. 1963.
- Untersuchungen uber die Chloroplastenbewegung bei Vaucheria sessilis.
Protoplasma 56: 495 - 520.
- Haupt, W. 1982.
- Light mediated movement of chloroplasts.
Ann. Rev. Plant Physiol. 33: 205 - 233.
- Hay, M. E., V. J. Paul, S. M. Lewis, K. Gustafson, J. Tucker and R. N. Trindell. 1988.
- Can tropical seaweeds reduce herbivory by growing at night? Diel patterns of growth, nitrogen content, herbivory and chemical versus morphological defenses.
Oecologia 75: 233 - 245.
- Jeffrey, S. W. 1968.
- Pigment composition of Siphonales algae in the brain coral Favia.
Biol. Bull. 135: 141 - 147.
- Kamiya, N. 1986.
- Cytoplasmic streaming in giant algal cells: A historical survey of experimental approaches.
Bot. Mag. 99: 444 - 467.
- Koop, H-U., R. Schmid, H-H. Heunert and B. Milthaler. 1978.
- Chloroplast migration; a new circadian rhythm in Acetabularia.
Protoplasma 97: 301 - 310.
- Mariani-Colombo, P. 1978.
- An ultrastructural study of thallus organisation in Udotea petiolata.
Phycologia 17: 227 - 235.
- Menzel, D. 1985.
- Fine structure study on the association of the caulerpalean plastid with microtubule bundle in the siphonalean green alga Chlorodesmis fastigiata (Udoteaceae).
Protoplasma 125: 103 - 110.
- Menzel, D. 1988.
- How do giant plant cells cope with injury. The wound response in siphonous green algae.
Protoplasma 144: 73 - 91.
- Menzel, D. and M. Schliwa. 1986 a.
- Motility in the siphonous green alga Bryopsis. I. Spatial organisation of the cytoskeleton and organelle movements.
Eur. J. Cell. Biol. 40: 275 - 285.
- Menzel, D. and M. Schliwa. 1986 b.
- Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organised arrays of both microtubules and actin filaments.
Eur. J. Cell. Biol. 40: 286 - 295.
- Nultsch, W, J. Pfau and U. Ruffer. 1981.
- Do correlations exist between chromatophore arrangement and photosynthetic activity in seaweeds?
Mar. Biol. 62: 111 - 117.
- Paul, V. J. and W. Fenical. 1983.
- Isolation of halimedatrial: chemical defense adaptation in the calcareous reef-building alga Halimeda.
Science 221: 747 - 749.
- Paul, V. J. and W. Fenical. 1984.
- Bioactive diterpenoids from tropical marine algae of the genus Halimeda.
Tetrahedron 40: 3053 - 3062.
- Paul, V. J. and K. L. Van Alstyne. 1988.
- Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimedaceae; Chlorophyta).
Coral Reefs 6: 263 - 269.
- Stark, L. M., L. Almodova and R. W. Krauss. 1969.
- Factors affecting the rate of calcification in Halimeda opuntia (L.) Lamouroux. and Halimeda discoidea Decaisne.
J. Phycol. 5: 305 - 312.
- Trench, R. K., M. E. Trench and L. Muscatine. 1972.
- Symbiotic chloroplasts: their photosynthetic products and contribution to mucus synthesis in two marine slugs.
Biol. Bull. 142: 335 - 349.
- Trench, R. K., J. E. Boyle and D. C. Smith. 1973.
- The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. 11. Chloroplast ultrastructure and photosynthetic carbon fixation in E. viridis.
Proc. Roy. Soc. Lond. B 184: 63 - 81.
- Williamson, R. E. 1986.
- Organelle movement along actin filaments and microtubules.
Plant Physiol. 8: 631 - 634.
- Zurzycki, L, T. Walczak, H. Gabrys and J. Kajfosz. 1983.
- Chloroplast translocations in Lemna trisulca L. induced by continuous irradiation and by light pulses. Kinetic analysis.
Planta 157: 502 - 510.










