Please note that this material is copyright to the publishers of Biological Rhythm Research and to the authors
Not to be reproduced in any way without permission except for personal study

Biological Rhythm Research, 1995, Vol. 26. No. 1. pp. 48-54
Studies on Halimeda. V.
Effect of Temperature on Chloroplast Migration in
this Siphonous Green Alga*
Edward A. Drew and Kay M. Abel
Australian Institute of Marine Science. PMB No 3, MC, Townsville, Q 4810. Australia.
* Publication No 697 from the Australian Institute of Marine Science.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
ABSTRACT
The effect of temperatures between 15 and 30oC on the daily cycle of chloroplast migration in Halimeda distorta and H tuna was determined from changes in segment pigmentation recorded by time-lapse videography throughout the experiments. An un-named opuntioid species was also tested between 20 and 35oC. Both the daily pattern and the amplitude of change in surface pigmentation. which were sustained for at least 5 days at 25oC. were unchanged at the higher temperatures. At 20oC the amplitude was considerably reduced but cyclical changes in surface pigmentation continued to occur throughout the 3-day low temperature period. However, at 15oC even greater reduction in amplitude was observed together with a reduced rate of paling at the onset of darkness and absence of pre-dawn re-greening. Furthermore, at 15oC all daily changes in surface pigmentation had ceased by the second day in H tuna and by the third day in H distorta. These effects of lower temperatures were reversed when the plants were returned to 25oC, although after the 15oC treatment of H tuna the amplitude of the change in surface pigmentation in two of the three replicate plants was small on the first night back at 25oC whilst the third plant lost pigmentation progressively and was completely white. and apparently dead, two days later.
Abstracting keywords: Halimeda, coenocytic green alga, chloroplast movement, elevated temperature, reduced temperature, cytoskeleton.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
INTRODUCTION
Large scale chloroplast migrations are known to occur in some siphonous green algae including Caulerpa (Dawes & Barilotti, 1959), Acetabularia (Koop et al., 1978) and Halimeda (Drew & Abel, 1990, 1992). The daily migrations in all three algae involved basipetal migration in darkness, and followed endogenous, circadian rhythms as demonstrated by their persistence for at least 7 days in Acetabularia in continuous light, and at least 3 days in Caulerpa and seven days in Halimeda in continuous dark.
The structural basis of chloroplast migration in Halimeda is considered to be a microtubule/actin cytoskeleton (Drew & Abel, 1990) as described in another siphonous green alga, Bryopsis, by Menzel and Schliwa (1986 a,b). The easily recorded changes in surface pigmentation, which are directly related to chloroplast movement in Halimeda (Drew & Abel. 1990), provide a useful system to quantify environmental effects on the cytoskeleton.
In this paper we report experiments to determine the in vivo effect of temperature on chloroplast movement.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
MATERIALS AND METHODS
Experiments with both H distorta (Yamada) Colinvaux and H tuna (Ellis and Solander) Lamouroux were carried out using time-lapse colour videography as described by Drew & Abel (1992) for H distorta, except that only the terminal three segments of branches of the larger-segmented H tuna were used, compared with six segments in H distorta. Some temperature experiments were also carried out with the time-lapse black and white photography methods and the Halimeda sp. used by Drew & Abel (1990). A range of incubation temperatures between 15 and 35oC � 0.2oC was achieved by keeping the dark cool-room at 13oC and heating the waterbath. Temperature changes were initiated before noon and had always stabilised before the start of the next dark period.
The changes in surface pigmentation reported represent movement of chloroplasts away from (paling) or back into (re-greening) the surface utricles of Halimeda segments as demonstrated by Drew & Abel (1990) using light microscopy.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
RESULTS AND DISCUSSION
The results for 3-day temperature excursions above 25oC (H tuna) and below 25oC (H tuna and H distorta) are illustrated in Figures 1. Data for H sp. are not illustrated.
Halimeda distorta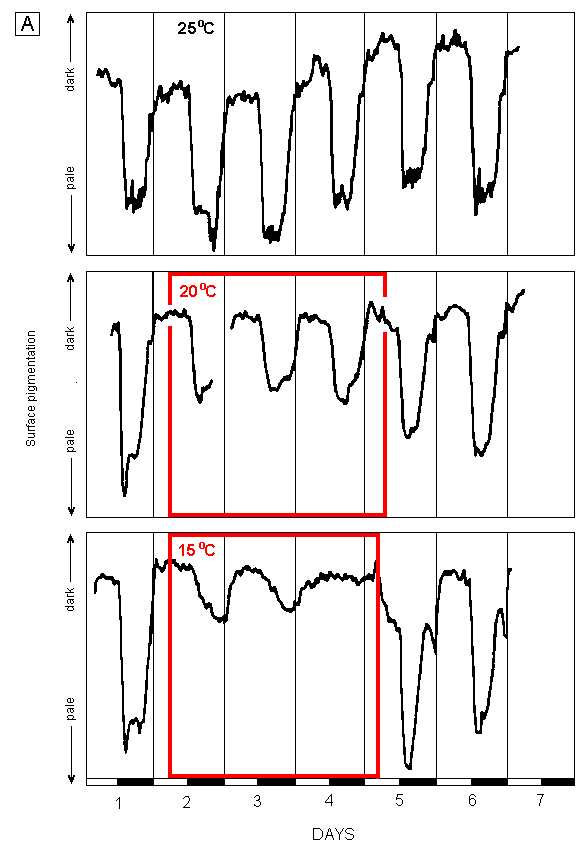
|
Halimeda tuna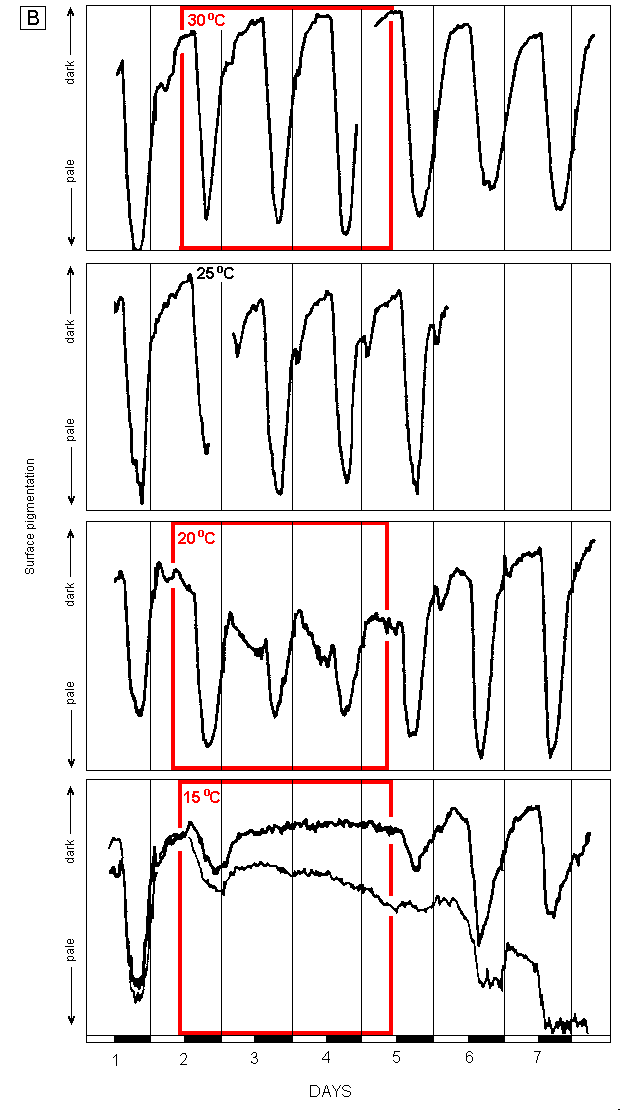
|
Fig. 1. Effects of temperatures between 15oC and 30oC on changes in surface
pigmentation of H distorta (A) and H tuna (B). |
Periods at temperatures other than 25oC are enclosed in labelled boxes. Irradiance during light periods (indicated by open bars on bottom axes) = 100 uE m-2 s-1. Graphs represent mean values for two segments on different plants. All the other segments on the three replicate plants used in each experiment behaved similarly to those shown in the graphs except in the 15oC treatment for H tuna where the thick line is representative of the segments on two plant, and the thin line is representative of those on the third plant. |
The normal response
All experiments began with one overnight period at 25oC, and those with H distorta and H tuna finished with 2 or more days back at that temperature. All plants demonstrated the normal response cycle described by Drew & Abel (1990), although the change from green to nearly white in H tuna took about 4 hours, compared with only 2 hours in H distorta and H sp. This may reflect the lower level of calcification of H tuna, rather than actual slower chloroplast movement, as the former would allow the chloroplasts to remain "visible" for longer. Predawn greening began at about the same time in all three species, so the period of minimum pigmentation, which lasted several hours in H distorta and H sp., was extremely short in H tuna.
All subsequent references to normal cycles of change and levels of pigmentation refer to those observed at 25oC on the first day of each experiment and are the same as previously reported by Drew and Abel (1990, 1992).
Elevated temperatures
H distorta was not exposed to temperatures above 25oC but H sp. was exposed to 35oC for 4 days and H tuna to 30oC for 3 days. There were no quantitative or qualitative differences in the cycles of pigmentation change in either species compared with that at 25oC.
Reduced temperatures
In both H distorta and H tuna, the cyclic pattern of pigmentation change was unchanged during a 3-day period at 20oC and there was no change in the rates of paling after dark and re-greening still began well before dawn. However, at 20oC the amplitude of paling was reduced by about half. H distorta reached full pigmentation during the day throughout the experiment whereas in H tuna, because complete paling occurred on the first night but regreening on all three mornings only proceeded to about half the normal level, the plants were paler than usual throughout the period at 20oC. H sp. was exposed to 20oC for only 1 day. As with H distorta, it only paled to about half the normal amount that night. However, it did not show any pre-dawn re-greening the following morning and the experiment was terminated 2 hours after dawn at which time it was still re-greening rapidly but had not yet reached the normal daytime pigmentation.
Cooling to 15oC eventually eliminated the diurnal changes in pigmentation in both species tested, although they differed in the time it took for this effect to be fully manifested. The amplitude of paling in both species was reduced to only about 25% of normal and the rate of paling was also considerably slower. Both species then re-greened to their normal level the next morning, although there was no indication of pre-dawn re-greening in either. Thereafter, H tuna showed no further pigmentation change during the remaining 2 days at 15oC whereas H distorta paled to some extent on the second night, re-greened after dawn the next day but did not become pale that night.
Recovery at 25oC
H tuna continued to follow the normal cycle of diurnal pigment changes for at least 3 days when returned to 25oC after 3 days at 30oC.
Both H distorta and H tuna returned to their normal cycles for at least 2 and 3 days, respectively, after 3 days at 20oC.
The only apparent effect of 3 days at 15oC on H distorta was the initiation of pre-dawn greening earlier than before, an effect which was maintained for at least 2 days at 25oC. However, H tuna showed a variety of effects of the 15oC treatment. Two of the replicates did exhibit both paling and pre-dawn re-greening at 25oC, although they paled to only about 25% of the normal amplitude on the first night and about 50% thereafter. The third replicate appeared to be severely damaged by the low temperature. Having progressively lost surface pigmentation towards the end of the 15oC treatment, it did not pale at all on the first night back at 25oC. It did pale considerably and rapidly on the second night of recovery, but showed no pre-dawn greening and did not return to even the lower pigmentation level of the day before. It then paled rapidly again the following night to become almost white and remained thus, apparently dead.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
CONCLUSIONS
The changes in surface pigmentation reported show that Halimeda is able to maintain a cyclic pattern of chloroplast migration at temperatures ranging between 20oC and at least 30oC, which is approximately the temperature range plants would experience in tropical seas. Some quantitative, but not qualitative, modification of the chloroplast migration cycle was detectable at 20oC. However, chloroplast migration was profoundly effected by only a few hours at 15oC and ceased altogether after only 1 or 2 days at that temperature, depending on species. Nevertheless. even exposure to 15oC for 3 days did not appear to cause irreversible damage, except to one H tuna plant, and a more or less normal cycle was usually re-established when the plants were returned to 25oC.
It should be noted that the maintenance of the normal pattern of chloroplast migrations over a significant temperature range (20-30oC) in these experiments does not constitute support or otherwise for the circadian nature of the endogenous rhythm involved, as that rhythm is expressed only in the dark (Drew and Abel, 1992). Light masks all its features except pre-dawn re-emergence, and the regular light/dark cycles used would, in any case. have served to re-entrain any such rhythm daily. To test the temperature compensation component needed, according to Schweiger and Schweiger (1977) to substantiate the truly circadian nature of an endogenous rhythm, experiments with Halimeda must be carried out in continuous darkness for several days at a range of temperatures which do not totally incapacitate the mechanism of chloroplast movement.
Assuming that the chloroplast migration cycle is essential for the metabolism and development of Halimeda, perhaps for one or more of the reasons discussed by Drew & Abel (1990), the plants investigated would be at a considerable disadvantage at temperatures below 20oC in the field with the operation of this cycle severely impaired. They would certainly be more susceptible to nocturnal molluscan grazers with the chloroplasts remaining at or closer to the surface at night. They would also be unable to populate their new segments with mature chloroplasts before dawn, resulting in delayed initiation of calcification and therefore increased susceptibility to other grazers such as fish. Reproduction might also be prevented because the large scale redistribution of cell contents into gametangia presumably requires a functional cytoskeleton.
Menzel & Schliwa (1986 b) reported that reversible disorganisation of the cytoskeleton and impairment of chloroplast movement both occurred rapidly at OoC in a temperate Bryopsis species, but not at 5oC. The progressive cessation of chloroplast movement we have observed in the tropical alga Halimeda during 1 to 2 days at 15oC could also have resulted from similar disorganisation of its microtubulel/actin cytoskeleton. Equivalent impairment of the functionality of the microtubules of the nuclear spindle at 15oC could be an important reason why this and related genera are restricted to tropical seas where temperatures seldom fall below 20oC.
GO TO Title-
Abstract-
Introduction-
Materials and Methods-
Results and Discussion-
Conclusions-
References
REFERENCES
- DAWES, C.J. and BARILOTTI. D.C. (1969):
- Cytoplasmic organisation and rhythmic streaming in growing blades of Caulerpa prolifera.
Am. J. Bot. 56: 8-15.
- DREW, E.A. and ABEL, K.M. (1990):
- Studies on Halimeda. III. A daily cycle of chloroplast migration within segments.
Bot. Mar. 33: 31-45.
- DREW, E.A. and ABEL, K.M. (1992):
- Studies on Halimeda. IV. An endogenous rhythm of chloroplast migration in the siphonous green alga. H. distorta.
J. interdiscipl. Cycle Res. 73 (2): 128-135.
- KOOP, H.-U., SCHMID, R., HEUNERT. H.-H. and MITLTHALER, B. (1978):
- Chloroplast migration, a new circadian rhythm in Acetabularia.
Protoplasma 97: 301-310.
- MENZEL, D. and SCHLIWA, M. (1986a):
- Motility in the siphonous green alga Bryopsis. I. Spatial organisation of the cytoskeleton and organelle movements.
Eur. J. Cell. Biol. 40: 275-285.
- MENZEL, D. and SCHLIWA, M. (1986b):
- Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organised arrays of both microtubules and actin filaments.
Eur. J. Cell. Biol. 40: 286-295.
- SCHWEIGER, H.-G. and SCHWEIGER. M. (1977):
- Circadian rhythms in unicellular organisms: an endeavour to explain the molecular mechanism.
Int. Rev. Cytol. 51: 315-342.


