Periodic Table Science
Wacky Patents New Scientist, Last Word, NS Plus
Space Sciene
SpaceDaily, Space Science Institute, The Nine Planets, Limerick-SEDS, Mars News,S.E.T.I
NASA: Today@NASA, Marshall Space Sciences, Ames Research Center?, Deep Space 1, Solar System Tour, ISS
Colonisation Projects
The Artemis Project, Permanent, The Planetary Society, Island One Society, Red Colony
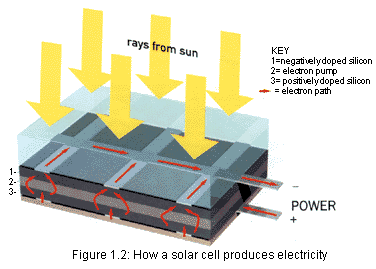 | 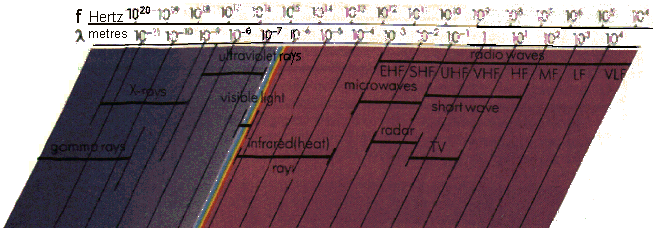 |
(m1 x m2)
F = G - -
d2
F=ma F=mg, where g= -9.2ms-2
where G is the gravitational constant of the universe,
g is the acceleration due gravity on earth, the moon has 1/6 of a g
m is the mass of the object
d is the distance between the two objects m1 and m2
There are in a few methods seen to get
Animated Gif of the Ozone layer over the world (1997)
Nasa's Mars schedule as described National Geographic August 1998
The launches will occur every 26 months, when Mars is in optimum position in relative to earth
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valancy | +1 | +2 | +3 -5 | +4 -4 | +5 -3 | -2 | -1 | 0 | |||||||||||
| 1 | 1 H |
2 He | |||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | |||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | |||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | |
| 6 | 55 Cs |
56 Ba |
* | 71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
** | 103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Uuu |
112 Uub |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
| *Lanthanoids | * | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
||||
| **Actinoids | ** | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
||||