|
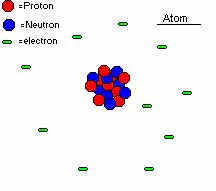
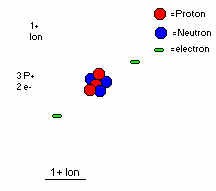
The reason why matter is called the fourth state of matter is because it is completely different from all of the other forms. It is most of the time extremely hot ionized gas particles. So what is ionized? If you have taken a chemistry course, I'm sure you have had it up to here with ions and so on. If not, let me explain it to you. As you know, an atom is composed of a dense center made of protons and neutrons. While the neutrons have no charge on them(thus neutral), the protons are positively charged. So if you were to have a magnet come into contact with one proton, the proton would stick on the negative side. So why don't all particles just cling to magnets? Well, there's another part to the atom, the electron. It is much smaller than the proton but has an equally strong opposite charge, negative. The little guy is just as strong chargewise as the big proton. This electron travels around the proton and neutron(called the nucleus) like our planets around the solar system. (Actually they don't, but this is a much simpler way to describe it.) Now what if you were to take one of these electrons away from the atom? WOW!! You have an ionized atom! Because you took away an electron(you can also add them in some cases)the atom now as a whole has a charge of 1+. Saa, all atoms always have an equal amount of electrons as protons, so an atom with five protons will have five electrons. That way the atom is neutral(good thing since we don't want everything sticking to magnets!). But take away an electron and the total charge is one proton extra, or 1+. This is called an ion. This one happens to be a positive one, but there are negative ones too. So since plasma is an ionized gas, is this ion plasma?
|
Not all ions are plasma. Plasma is an ionized gas, true, but it is only considered plasma when when most of the gas is ionized. Some plasma doesn't have ANY electrons attached to the nucleus! Seems easy right? Wrong. While it's easy to say it, it's much more difficult to do. When an atom loses an electron, it takes some amount of energy to do this, basically heat energy. But by doing so you've only taken off the electrons which comes off the easiest, or the one farthest off from the nucleus. In order to get to the second one, you have to heat the newly-made ion even more. Eventually you'll get to the second one and knock it away from the nucleus, but it takes more and more energy to do so. Atoms such as oxygen are stripped of their electrons only in the most extreme of conditions, such as in the sun or in a nuclear bomb explosion. This heat has to be kept up constantly, though, so that the electrons don't recombine with the nuclei. The reason why lies in the fact that temperature is really the measure of the speed of an atom, molecule or ion. These things are travelling at major speeds, even faster than our rockets...and this is just at room temperature! So the temperature increase causes the electron to have so much energy that they break free of the nucleus's hold, much like a rocket breaks free of the earth's gravity by putting so much energy out that it goes faster up than down. At this point whenever the electron tries to recombine it can't, because it's going too fast and its momentum is greater than the force that is being applied onto it by the nucleus(which is also flying at superman speeds). The ions and free electrons are the things we call plasma. "So what," you say, "what's this have to do with anything important? All it is, is a hot gas." Ha ha, read on oh ignorant one.
|
|
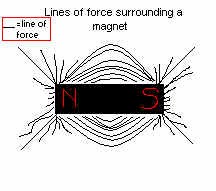
You have already fallen into the trap of thinking of plasma as a gas, it's not. Remember, this is the fourth stage of matter we're talking about. The main difference is as mentioned, plasma consists of completely or mostly ionized atoms with electrons swirling about. Another difference, though, is the strange way it is affected by magnetic fields. First of all, a curious property of plasma is that it resists separation of positive and negative ions. So if you have a magnet with a positive charge on one side, and a magnet with a negative charge on the other side, the plasma WILL NOT separate, where the electrons go onto the positive magnet and the positive ions go on the negatively charged magnet. It is not yet certain as to why this happens, but one hypothesis is that because there are an equal amount of protons and electrons in the plasma, the plasma as a whole is neutral, and thus acts as one entity by not separating. Instead, the plasma tends to flow along the lines of force. These lines of force are sort of like rubber bands, which pull the south and north ends of a magnet, or magnetic field together. These lines always stay in the same spot and are rarely moved. Even when moved they return to their original placs. Now, in a magnetic field, any electronically charged particles that pass through are strangely attached to the lines of force. A charged particle moving at right angles to the lines of force will feel a push, or force, that causes it to curve. The stronger the magnetic field, the greater the sideways force on the particle. If the particle energy and the field strength are right, the particle will be forced to follow a loop around one or more lines of force. The positive ion always loops around in the opposite direction to the negative electron. Another interesting thing about plasma is that it resists penetration by lines of force from the outside. Meaning once a plasma particle is on a line of force, it's going to stay on that specific one and won't go through other lines of force in order to resist the temptation of switching lines :) Even if the plasma is forced to shift or move in some way, it will bring the line with it, thus distorting it, but eventually going back into place. The relationship between the plasma and its magnetic field line of force is one that lasts for quite some time, and is not easily broken up. This cooperative movement of a field and its plasma is among the most important effects studied by plasma physicists.
|