|
| |
Chapter 2A. Getting around in Geology
KEY POINTS
The universe:
e.g. Hubble Telescope "Deep Field-South" Project [Oct. 1998]
- Focused on a part of the southern sky "About the size of a grain of sand held at
arm’s length."
- Amazingly: detected "12 billion Year old light" i.e. Galaxies 12 x
109 light
years away. (Where a light year = 10 trillion km = ~10 x 1012km) Which means that the
light source is approximately 12 x 1022 km away.
- "Big Bang" = only ~13-16 billion years ago, according to present estimates.
- Observed oddly shaped galaxies appearing to be "disorganized collections of loosely
bound lumps." i.e. galaxies in a very early stage of evolution.
Origin of the solar system:
- Age of solar system generally taken to be: 4.6 billion years from radiometric dating of
the moon and certain meteorites.
- Origin (see Fig. 2.2, P.24): Gravitational instability in an interstellar dust and
molecular cloud referred to as a nebula. E.g. Trifid Nebula. Generates an early star with
an interstellar disk; at least ~100 disks now observed. Orion Nebula: ~70 – 80% of
young stars have disks.
- Growth of planets by accretion of smaller objects, which had accreted, called
planetismals.
Can see the accretion process, the end of it, from the very old craters on the Moon and
Mercury.
Click here to view an article on the
Trifid Nebula.
Some meteorites contain small grains (~1 micron) with an isotope chemistry which
indicates that they originated before the solar system. Therefore: older than 4.6
billion years. (1 micron = 10-3 mm.)
e.g Al2O3
grains.
Graphite (C) spherules.
SiC (silicon carbide) and other carbides.
Silicon nitride etc.
Click here to view an article on
Stardust.
The Solar System: (Fig
2.3; p. 25)

The Earth System:
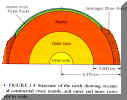
- Internal Structure (Fig 2.4; p. 25) :
Inner Solid Fe – Ni Core.
Outer Liquid Fe – Ni Core
Convective circulation generates the magnetic field.
Solid, but "plastic" (like ice in ice sheets) silicate mantle. Flows by
convection which causes plate tectonics.
Thin, silicate continental and oceanic "crusts".
The Ocean system.
Click here to view the five
interconnected spheres of the earth system.
Earth Materials (P.26)
- Elements, atoms and isotopes:
Key points:
- The essential basic component of matter is the element; elements are substances
that cannot be changed into other substances by "normal chemical" methods.
i.e..
without nuclear bombardment.
- As you know, all elements are defined by a particular atom type defined by a fixed
number of positively charged protons in the nucleus (= the atomic number).
- However, for many elements, the number of neutrons in the nucleus is variable. These
different types of nuclei's with the same number of protons, but different numbers of
neutrons are known as different isotopes of the element.
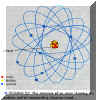
Isotopes:
Can be both:
- "Stable":
e.g. Hydrogen : 1 proton
Deuterium: 1 Proton + 1 Neutron
- "Unstable":
Undergo radioactive decay at different rates.
e.g. Tritium: 1 Proton + 2 Neutrons; decays with a ~ 12 year half life.
Radioactive Decay:
Can be:
A health hazard e.g. Radon.
Used for accurate dating: e.g. Carbon 14 decay; 5,730 year half life.
K decay to Argon.
Particularly: U isotope decay to different Pb (Lead) isotopes.
e.g. U 238 to Pb 206; 4.5 x 109 year half life.
Ions:
Ions are atoms that are charged positively or negatively depending on whether they have
lost, or gained, electrically negative electrons.
Ions can exist in solids (e.g. NaCl : salt – halite), aqueous solutions (e.g.
natural surface and ground waters), and also in the vapour phase ( => plasma).
Minerals:
Rocks consist of composites; mainly minerals, but also of glass and solidified organic
matter, principally.
Minerals are defined (p.28) as: naturally occurring, inorganic, crystalline*
substances, each with a narrow range of chemical compositions, and characteristic physical
properties.
*The word crystalline implies an exactly defined, ordered and repetitive internal
atomic or ionic structure
e.g. NaCl (Halite) at top of p.28.
- The beautiful natural faces of minerals reflect their ordered internal atomic/ionic
structure.
- Non-crystalline solids are referred to as amorphous:
e.g. glasses; precious opal.
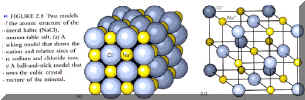
Rock Forming Minerals:
- Three of the most abundant crustal elements are oxygen (O), silicon (Si) and aluminum
(Al). Hence, the most important rock forming crustal minerals are: the silicates and
alumino-silicates:
- Their principal structural component is the (Si, Al) O4(4-) tetrahedral unit, as shown
in fig. 2.9, p. 29.
- These (Si, Al)O4(4-) tetrahedra are linked in 4 principal ways to generate a variety of
silicates/alumino-silicates with different properties: (See Fig. 2.9 (b), p.29)
Isolated.
Single + Double Chains
Sheets. e.g. micas.
3D networks e.g. quartz; feldspars.
Additional mineral groups include:
(ii) Oxides e.g. Hematite: Fe2 O3.
Magnetite: Fe3
O4.
(iii) Carbonates e.g. Calcite: Ca CO3 (in limestones).
(iv) Sulphides e.g. Pyrite: Fe S2.
(v) "Native" Elements: Uncombined. E.g. Gold, native
Copper.

Mineral Identification – Lab. I:
Using 5 main physical properties, plus a few others, many minerals can be identified,
or at least put in a group:
(i) Crystal Form (If Present):
The natural shapes of crystals can be used e.g. 4 simple examples in Fig. 2.12, P.33.
(ii) Hardness:
The simple Mohs hardness scale (1-10) is used (1812) – see table 2.2, P.32.
Note where a fingernail (~2.5), and a knife blade (~5) come.
10 = Diamond: Still the hardest known synthetic or natural substance.
1 = Talc; a very soft sheet silicate.
(iii) Cleavage:
Number of natural orientations, and natural orientation. Exceptionally important.
See. Fig. 2.11., p.33.
Note: some minerals have no cleavages, like quartz, but have a conchoidal fracture.
e.g. glass: break with concentric ripples.
(iv) Colour Appearance:
Actually, quite difficult to use; because it is quite variable. Use what you learn in
the lab.
Colour of powdered mineral: Known as STREAK, and has specific uses since
it can be consistent.
(v) Lustre:
"How a mineral reflects light" Not very clear, but:
e.g. Metallic – rich, reflected lustre.
Glassy, or vitreous – like glass. e.g. quartz.
Also: "oily"; "greasy"; "earthy".
(vi) Also Use:
e.g. (i) Magnetic, or not e.g. Magnetite.
(ii) Taste! e.g. Halite (NaCl).
|